One conclusion about the structure of the atom as a result of the gold foil experiment.
What is
The atom has a small, positive, dense nucleus
OR
The atom is mostly empty space
The ability of an atom to attract electrons.
What is electronegativity?
Cl2 --> Cl + Cl
The process occurring with the bonds and energy in the equation
What is bonds are broken and energy is absorbed (endothermic)?
Law that states that matter is neither created nor destroyed in a chemical reaction, meaning the mass of the reactants must equal the mass of the products.
What is the Law of Conservation of Mass?
A 10.0 gram sample of the hydrate is heated in a crucible until all water is driven off. Following the heating, the mass of the anhydrous remaining is 4.9 grams. This is the percent by mass of water in the original hydrate.
What is 51% H2O?
This type of energy remains constant during any phase change.
What is average kinetic energy (or temperature)?
These are atoms of the same element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers
What are isotopes?
Different forms of the same element. They have different structures and different properties.
What are allotropes?
In this type of bond, electrons are shared equally between two atoms
What is a nonpolar covalent bond?
In this specific type of reaction, a single compound breaks down into two or more simpler substances
What is a decomposition reaction?
The total mass of water formed when 12 grams of hydrogen reacts completely with 64 grams of oxygen
2H2 + O2 → 2H2O
What is 76 grams of water?
This term describes a substance changing directly from a solid to a gas without ever becoming a liquid
What is sublimation?
This phenomenon occurs when an electron releases energy in the form of light as it falls from an excited state back down to its ground state
What is a bright-line spectrum (or emission spectrum)?
According to the "Octet Rule," atoms are in their most stable state when they possess this many valence electrons
What is 8?
The Lewis Structure for PF3
[Teacher Approves Drawing]
The name of Pb(SO4)2
What is lead(IV) sulfate?
Based on the equation N2 + 3H2 --> 2NH3
This is the total number of moles of Ammonia produced if you start with 12.0 moles of Hydrogen?
What is 8 moles?
This is the heat absorbed when 75 g of water warms from 20°C to 40°C.
What is 6,270 joules?
This is the ground state electron configuration for an atom of Silicon if you use the spdf method
What is 1s2 2s2 2p6 3s² 3p2?
As atomic number increases across Period 3, atomic radius generally does this.
What is decrease?
The bond polarity and molecular polarity of CO2.
What is polar bond and non-polar molecule?
These are the correct coefficients needed to balance the following equation:
__NH4Cl + __Hg2(C2H3O2)2 → __NH4C2H3O2 + _Hg2Cl2
What is 2,1,2,1?
A compound has the empirical formula CH2. If its actual molecular mass is 42 g/mol this is its molecular formula.
What is C3H6 ?
 Draw a diagram of a mixture of water and chlorine both in the gaseous phase
Draw a diagram of a mixture of water and chlorine both in the gaseous phase
[Teacher approved drawing]
The net charge of an atom that has 13 protons, 10 electrons, and 14 neutrons. The atomic symbol and ion name?
What is +3? What is 27Al+3, a cation?
These six elements are lustrous but considered to be brittle
What are the metalloids, B, Si, Ge, As, Sb and Te?
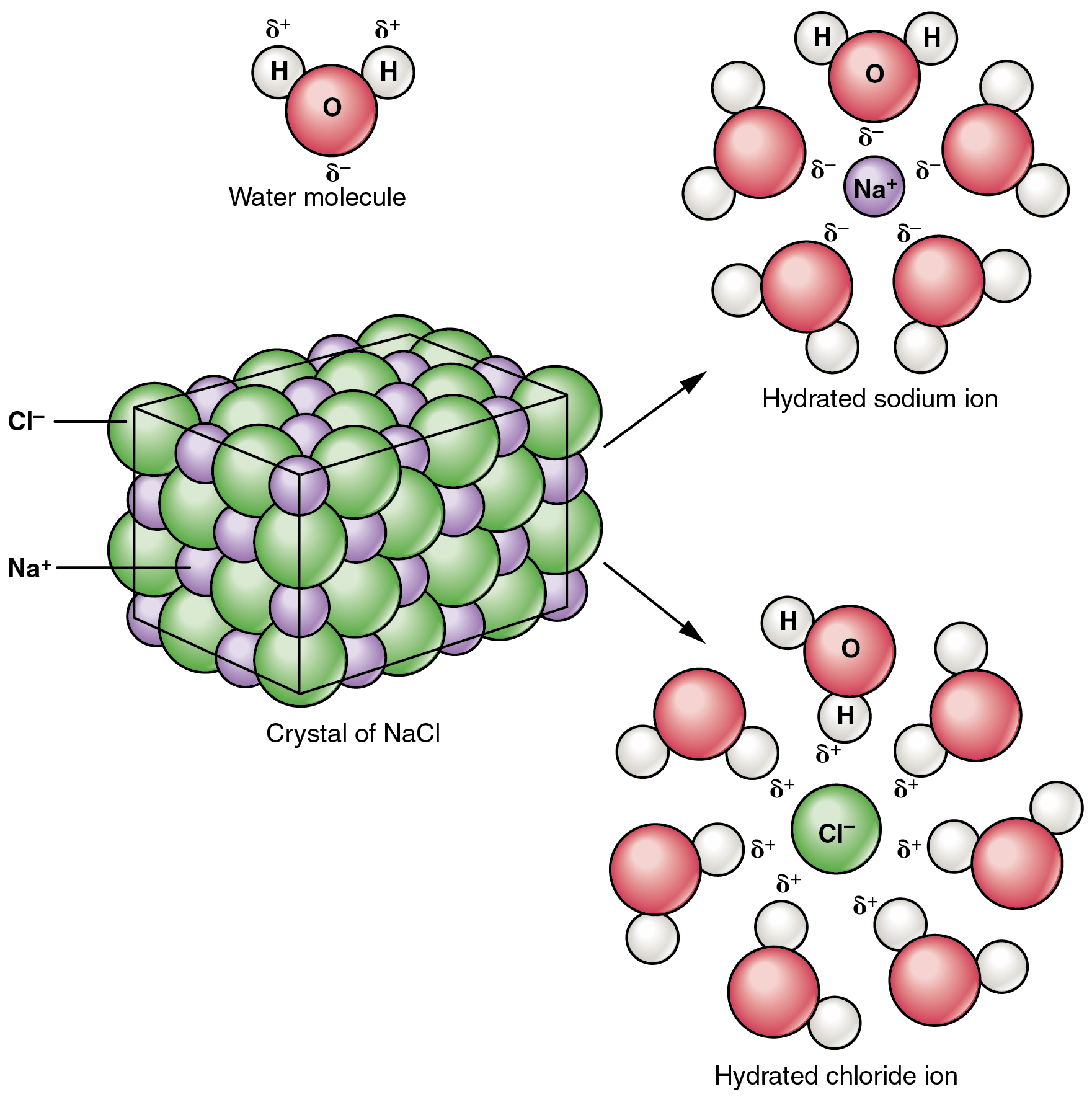 This type of attraction explains why ionic substances can dissolve in water; it occurs when the partial charges of polar water molecules surround and attract individual ions.
This type of attraction explains why ionic substances can dissolve in water; it occurs when the partial charges of polar water molecules surround and attract individual ions.
What is molecule-ion attraction (or Ion-Dipole force)?
This is the correct chemical formula for the compound Magnesium Phosphate
What is Mg3(PO4)2 ?
Based on the balanced equation
2 KClO3 --> 2 KCl + 3 O2
Calculate the total mass in grams of Oxygen produced if 245 grams of KClO3 is fully decomposed. (GFMs: KClO3= 122.5 g/mol, O2 = 32.0 g/mol)
What is 96 grams?
Explain, in terms of particle distribution, why NaCl(aq) is a homogenous mixture?
The particles are distributed evenly or uniformly.