List the prefixes from largest to smallest
Kilo
Hecto
Deka
Deci
Centi
Milli
How many sig figs are in 1.080?
4
What is the formula and units for Density?
D=M/V
g/mL or g/cm3
Define independent and dependent variables.
independent- variable changed
dependent- variable measured
What is pressure?
gas particle collisions against container walls
Which is the larger value? 7g or 698mg
7g
Name two of the three types of numbers that have unlimited sig figs and therefore don't count towards sig figs in calculations?
Counting numbers
Conversion factors
integral numbers
Which box has the lowest density?
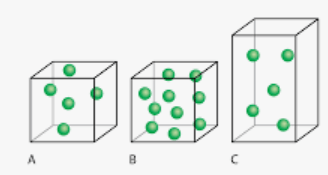
Box C
What type of relationship does the graph show?
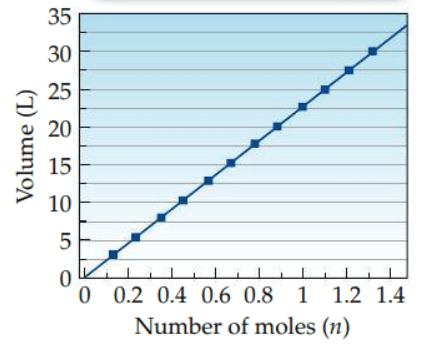
Direct
A cylinder equipped with a moveable piston has an applied pressure of 4.0 atm and a volume of 6.0 L. What is the volume of the cylinder if the applied pressure is decreased to 1.0 atm?
24 L
Give the correct measurements for this instrument.
A. B.
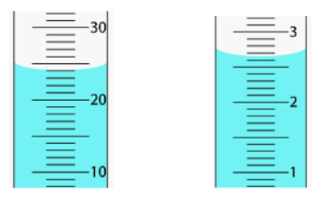
a. 24.0 mL
b. 2.65 mL
Perform each calculation to the correct number of sig figs.
1.01 x 0.12 x 53.51 ÷ 96 =
0.068
If you blow up a balloon and then let out a quarter of the air from the balloon, what happens to the density of the balloon from when it was the original size to when the size was reduced?
It stays the same
What law does this graph represent?
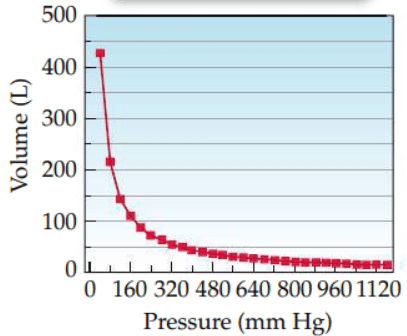
Boyle's Law
A sample of gas has an initial volume of 158 mL at a pressure of 735 mmHg and a temperature of 34oC. If the gas is compressed to a volume of 108 mL and heated to a temperature of 85oC, what is its final pressure in mmHg?
1.25 x 103 mmHg
Alka Seltzer (13.5 g) is placed in a flask containing 104.7 g of water. A balloon is placed over the top of the flask and inflates. What is the total mass of the products after 5 minutes? What would happen to the mass if the balloon is taken off the flask? Why?
118.2g
mass would decrease because gas would escape into the surroundings
Perform the calculation to the correct number of sig figs.
0.765 - 3.449 - 5.98 =
-8.66
What is the density of a piece of wood that has a mass of 25 grams and a volume of 294 cm3? Would the piece of wood sink or float on water?
0.085 g/cm3, it would float
Which gas variables are changing and how are they changing?
Volume decreasing
pressure increasing
Calculate the volume occupied by 0.845 particles of nitrogen gas at a pressure of 1.37 atm and a temperature of 315 K.
16.0 L
Convert 0.23 kg to dg and write the answer in scientific notation.
2.3 x 103 dg
Perform the calculation to the correct number of sig figs.
19.667 - (5.4 x 0.916) =
14.7
What is the mass of a cylinder of lead that is 2.5 cm in diameter, and 5.5 cm long? The density of lead is 11.4 g/mL
mass= 307.78 g
What is the x-intercept value and what does it mean?
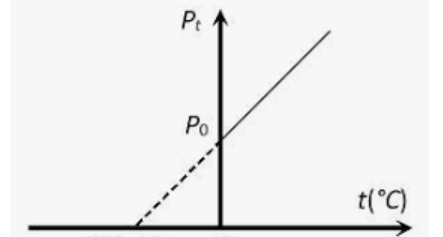
-273 oC and absolute zero (no motion in particles)
A mixture of helium, nitrogen, and oxygen has a total pressure of 752 mmHg. The partial pressures of helium and nitrogen are 234 mmHg and 197 mmHg, respectively. What is the partial pressure of oxygen in the mixture?
321 mmHg