The faster the molecules move, the ________________ the temperature.
Higher
__________________ occurs when water vapor in the air slows down sufficiently to become a liquid.
Condensation
A pot of water is placed on a hot stove. Small bubbles begin to appear at the bottom of the pot. The bubbles rise to the surface of the water. What are the bubbles made of?
Gaseous water molecules or water vapor
What is phase change A called?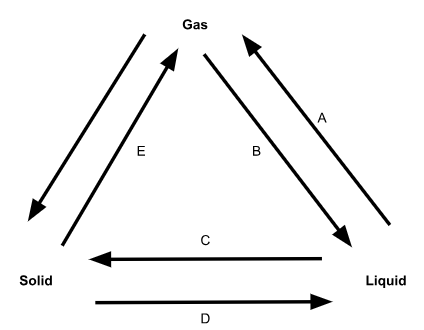
Evaporation
Why do different substances have different odors?
They have different molecules. They could be made from different atoms, different number of atoms or atoms arranged in different ways.
Temperature is a measurement of the average __________________________ of molecules.
Speed
When a substance is heated, the molecules that make up the substance move ____________________.
Faster
A pot of water on the stove begins to boil rapidly. A glass lid is placed on the pot and water droplets begin forming on the inside of the lid. How did those water droplets get there?
Steam rose up to the lid then cooled down and moved closer together to form water molecules. This is called condensation.
What is phase change B called?
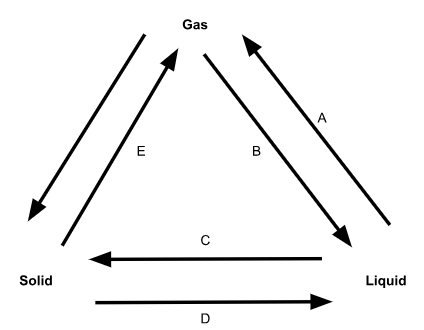
Condensation
What are two types of detectors we have used to prove that odors are in the air?
We used our nose to detect odors. We used indicator or pH paper to detect odors.
As temperature increases, the volume of gas _________________________.
Increases
In a solid, molecules stay in place and can only ________________.
Vibrate
What happens to water molecules when water is cooled from 50 degrees fahrenheit to 32 degrees fahrenheit and freezes?
The molecules move more slowly and get closer together.
What is phase change C called?
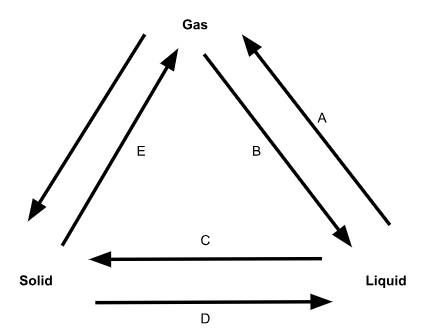
Freezing
Why do balloons in a warm room stay inflated but ballons in a cold room begin to deflate?
When the temperature of a gas increases so does the volume because the particles move faster and spread out more. When the temperature of a gas decreases so does the volume because the particles slow down and move closer together.
As temperature decreases, the volume of gas ____________________________.
Decreases
________________ is when a substance changes from a solid state into a liquid state. The opposite process is called _______________________.
Melting and freezing
What water changes phases from ice to liquid water to water vapor, do the water molecules themselves change?
No, they stay the same size and are still H2O.
What is phase change D called?
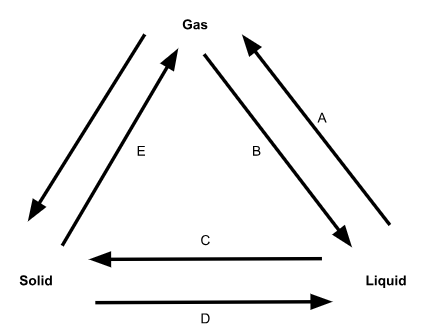
Melting
Are the particles on the left in a cold or hot room? How are they different than the model on the right?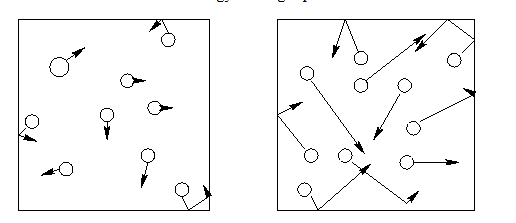
Cold room. They are moving slower and are closer together.
_________________________ occurs when molecules at the surface of a liquid gain enough energy to enter the gaseous state.
Evaporation
Substances can change from a solid state to a gaseous state, and this is called _______________________.
Sublimation
What is water in its gaseous phase called?
Water Vapor
What is phase change E called?
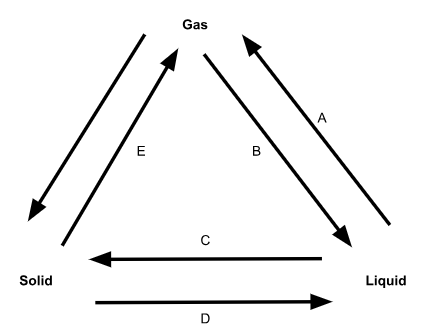
Sublimation
Are the particles on the right in a cold or hot room? How are they moving differently than those on the left?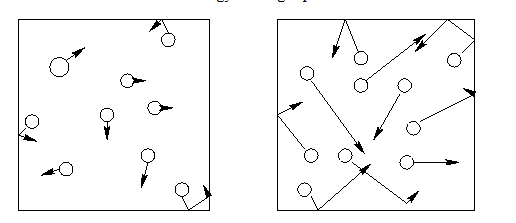
Hot room. They are moving faster and are more spread out.