The only neutral subatomic particle.
Neutron
The outermost electrons in an atom responsible for bonding.
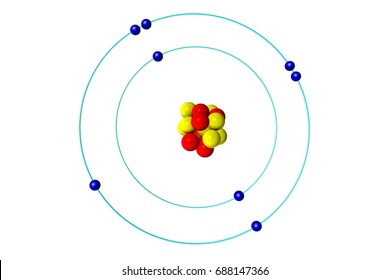
Valence Electrons
A bond where electrons are shared.
Covalent Bond
One way to increase the speed of a reaction
Increase Surface Area, Temperature, Agitation, Concentration of Reactants.
Add a Catalyst
One dozen is 12 things, One mole is...
Avogadro's Number of things
6.02 x 1023 things
3 Si + 2 N2 → Si3N4
According to the balanced equation 9 moles of Si produces __ moles Si3N4
3
(9 mol Si) x (1 mol Si3N4 /3 mol Si) = 3 moles Si3N4
According to the octet rule, atoms want this number of valence electrons to get a stable noble gas configuration.
8
A nuclear reaction where two or more nuclei come together to make a larger nucleus.
Fusion
The most electronegative element in the periodic table.
Fluorine
Cations are positive, _____ are negative.
Anions
This is the profile of a ____ reaction.
Endothermic
20 moles of Helium weighs...
80 grams
mass = moles x molar mass
mass = 20 mol x 4 g/mol = 80
CH4 + 2 O2 → CO2 + 2 H2O
In the reaction above 10.0 g CH4 makes 27.4 g CO2, while 10.0 g O2 makes 6.88 g CO2. The limiting reactant is...
O2
Oxygen
An element that is shiny silver solid falls on the floor and breaks. This element is most likely...
Metalloid / Semimetal
This is an example of ___ decay.
Alpha
The common charge of an element in group 15
-3
The chemical formula for Copper (III) Chloride
CuCl3
The two products of the complete combustion reaction of organic molecules.
CO2 and H2O
Carbon dioxide and Water
The Volume of 1 mole of gas at STP....
22.4 L
PV = nRT
STP = 273 K & 1 atm
(1 atm x V) = (1 mol x 0.0821 Latm/Kmol x 273 K)
V = 22.4 L
If the pOH of a solution is 10,the pH of the solution is..
4
pH + pOH = 14
pH + 10 = 14
pH = 14 - 10 = 4
Catalyst increase the rate of the reaction by lowering the ______
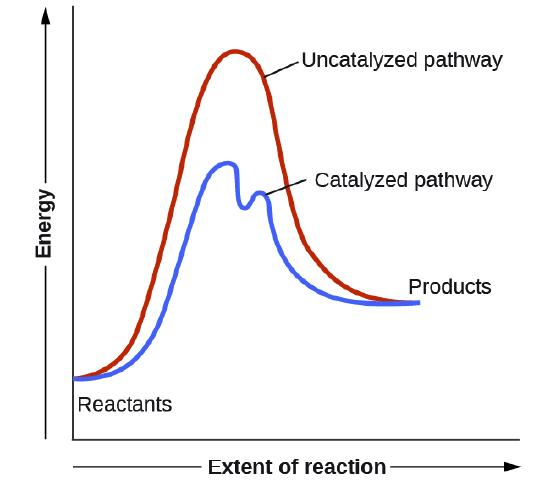
Activation Energy (Ea)
The number in the upper left corner of the element symbol.
?X
The Mass Number
Elements in group 18 are known as...
Noble Gases
The name of this molecule PCl5
Phosphorous Pentachloride
These numbers will balance the equation…
__N2 + __F2 → __NF3
1, 3, 2
1 N2 + 3 F2 → 2 NF3
The empirical formula of C6H4O2 is...
C3H2O
Simplify the subscripts by dividing by the Least Common Factor of 2.
The moles of Cl- in solution when 5 moles of Aluminum Chloride (AlCl3) ionize in water
15 moles
AlCl3 → Al3+ + 3 Cl-
(5 mol AlCl3 /1) x (3 mol Cl-/1 mol AlCl3) = 15 moles Cl-
According to the ideal gas law PV equals...
"nRT"
moles x gas constant x Temperature
The name of the element that balances this equation.
3717Cl → ? + 0-1e
Argon
This shows electron configuration for...
1s22s22p63s23p64s13d10
Copper
The strongest intermolecular forces found in water.
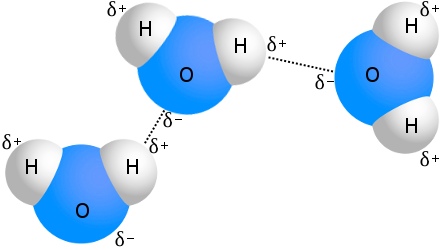
Hydrogen Bonds
The missing product of this acid base neutralization is…
2 HCl + Ba(OH)2 → ? + 2 H2O
Barium Chloride or BaCl2
When 10 mL of 2 M solution is diluted to 40 mL, the concentration of the dilute solution is..
0.5 M
M1V1 = M2V2
10 x 2 = M2 x 40
M2 = 20 / 40
The percent yield is 50%, the theoretical yield is 60 g, so the actual yield is...
30 g
An atom with 8 protons, 7neutrons, 10 electrons has a ___ charge.
-2
Negative two charge
Charge = 8 protons - 10 electrons = -2