What is the definition of Matter
Anything that has mass or volume
-or-
The stuff that makes up everything
This subatomic particle is negatively charged and orbits the nucleus.
Electron
This state of mater has definite shape and volume.
Solids
What is the chemical formula for 2 separate Nitrogen atoms floating next to each other?
2N
Are reactants to the left or right of the yield arrow?
Left
What is the definition of Mass
The amount of Matter in an object
This Element Symbol is located in Period 5 group 16 (6A)
Te
Phase changes are caused by the gain or loss of this type of energy.
Thermal (heat)
Is this an molecule or a compound?
Molecule
Balance the following equation
2H2 + __O2 >> ___H2O
2H2 + O2 >> 2H2O
The following term is used to measure fluids in milliliters or centimeters cubed.
Volume
What is the formula for calculating the number of neutrons in an atom?
Average Atomic Mass - Atomic Number
Is mixing melted butter with mashed potatoes a physical or chemical change?
Physical
How many elements are in NaHCO3 (soda!)
4
Describe the Law of Conservation of Mass and Matter.
Matter cannot be created or destroyed.
What is the Density of an object that has a mass of 123 g and a volume of 456 mL (round to the nearest hundredth)
0.27 g/mL
What group number is this element located in?
Group 16 or 6(A)
What phase change describes liquid magma turning to solid rock?
Freezing
What is the difference between a molecule and compound? (include a drawing of each)
Molecules = 2 or more atoms bonded together.
Compounds = 2 or more atoms bonded together and they are different elements.
Balance the following Equation
___C6H12O6 >> ___C2H5OH + ___CO2
C6H12O6 >> 2C2H5OH + 2CO2
Honey has a density of 1.4 g/cm3.
Would a glass marble with a mass 18.5 g and volume of 12.5 cm3 float in a bowl of Honey? (show your work)
No - 1.48 g/cm3 > 1.40 g/cm3
What element is this?
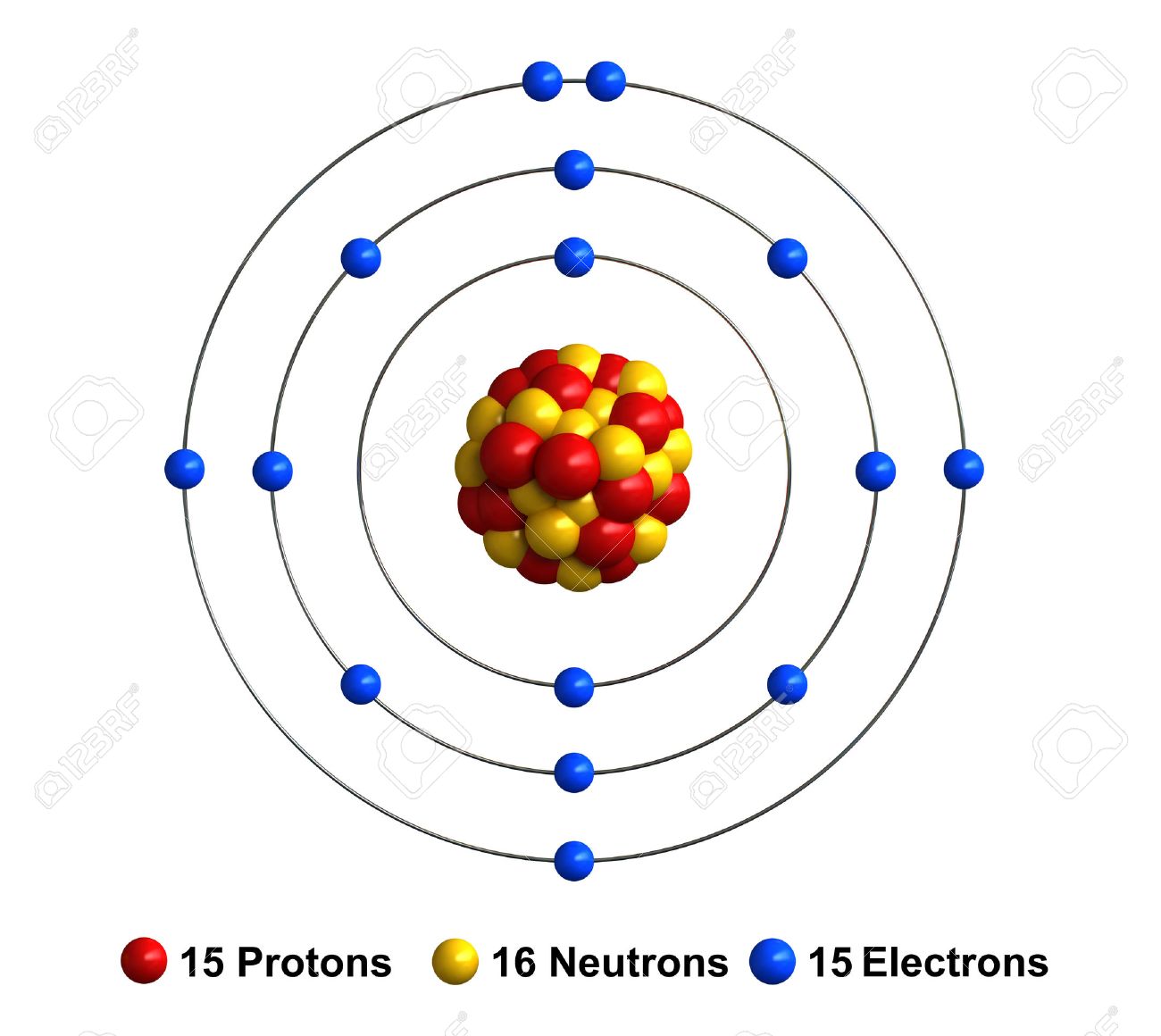
Phosphrous
Is the process of fermentation an example of a physical or chemical change? (explain why!)
Chemical
How many total atoms are in 3 molecules of C8H10N4O2 (caffeine) ?
72 atoms
Does the equation below follow the Law of Conservation? Why or why not?
2C4H10 + 12O2 >> 8CO2 + 10H2O
No, because matter was created (oxygen)