Of the following: sucrose, distilled water, seawater, and sodium chloride, it is the only example of a mixture.
What is Seawater?
This is the method of separation that would work best to separate a solid from a liquid in a heterogeneous mixture.
What is filtration?
These are the names of the three sub-atomic particles found in an atom along with their respective charges.
What are Proton (+), Electron (-), Neutron (0)?
This is the primary difference between isotopes of the same element, and ions of the same element.
What is the number of neutrons for isotopes and number of electrons for ions?
This is the maximum number of electrons that can be placed in a 4p subshell.
This is the temperature in Kelvins when the temperature is -185 oC
What is 88 K?
This is the method of separation primarily used to separate two liquids that are miscible within each other.
What is distillation?
These are the names of the elements with the numbers of protons equaling 32, 47, and 15.
What are Germanium, Silver, and Phosphorus?
This is the number of electrons in 3517Cl-1.
What is 36?
This is the number of types of orbitals and what they are called?
What is 4 --> s, p, d, f?
This is what the energy is being used to do at the 3rd point on the heating curve of ice as shown below.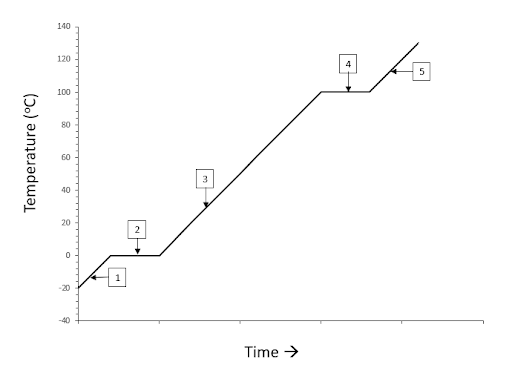
What is raising the temperature of liquid water until it reaches the vaporization point?
This method of separation could be used to determine the purity of pharmaceuticals.
What is paper chromatography?
True or False: Two atoms of the same element can have different numbers of protons?
What is false?
Of the three following properties, these are the only two that are true for two isotopes of the same element.
1. Have the same atomic number.
2. Have the same physical properties.
3. Have the same chemical properties.
What are #1 and #3?
This is the electron configuration of the Cobalt ion, Co+2.
What is 1s22s22p63s23p64s23d7?
This is what the phase change freezing is when referring to endothermic or exothermic.
What is exothermic?
This is one way that you could separate salt granules from sand granules.
What is dissolution?
These are the number of protons, electrons, and neutrons that a neutral atom of Osmium have.
What are 76 protons, 76 electrons, and 114 neutrons?
This is the number of neutrons in the isotope Palladium - 107.
This is the electron configuration for Yttrium using the noble gas shortcut.
What is [Kr]5s24d1?
These are the two states of matter that have no rigid shape.
What are liquids and gases?
True or false: separation methods only utilize either physical or chemical properties, never both.
What is false?
This is the conclusion that Ernest Rutherford came to when he conducted the gold foil experiment and found that when he blasted particles at a gold foil, some bounced back while some still went straight on through.
What is the idea that atoms are mostly empty space and that most of the mass is in the nucleus (in the middle)?
This is the isotopic symbol for an atom that has a mass number of 127, has 74 neutrons, and 54 electrons.
What is 12753I-1?
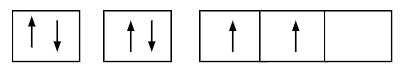
This is the orbital diagram for Carbon, C.
What is