Put into scientific notation 198,000,000.0
1.98x10^8
 Which month had the highest?
Which month had the highest?
August
The attraction between particles gives solids a definite.
Shape and Volume
The amount of energy required for a liquid at its boiling point to become a gas is _______.
Heat of vaporization
Convert 25 kilograms to grams
25000grams
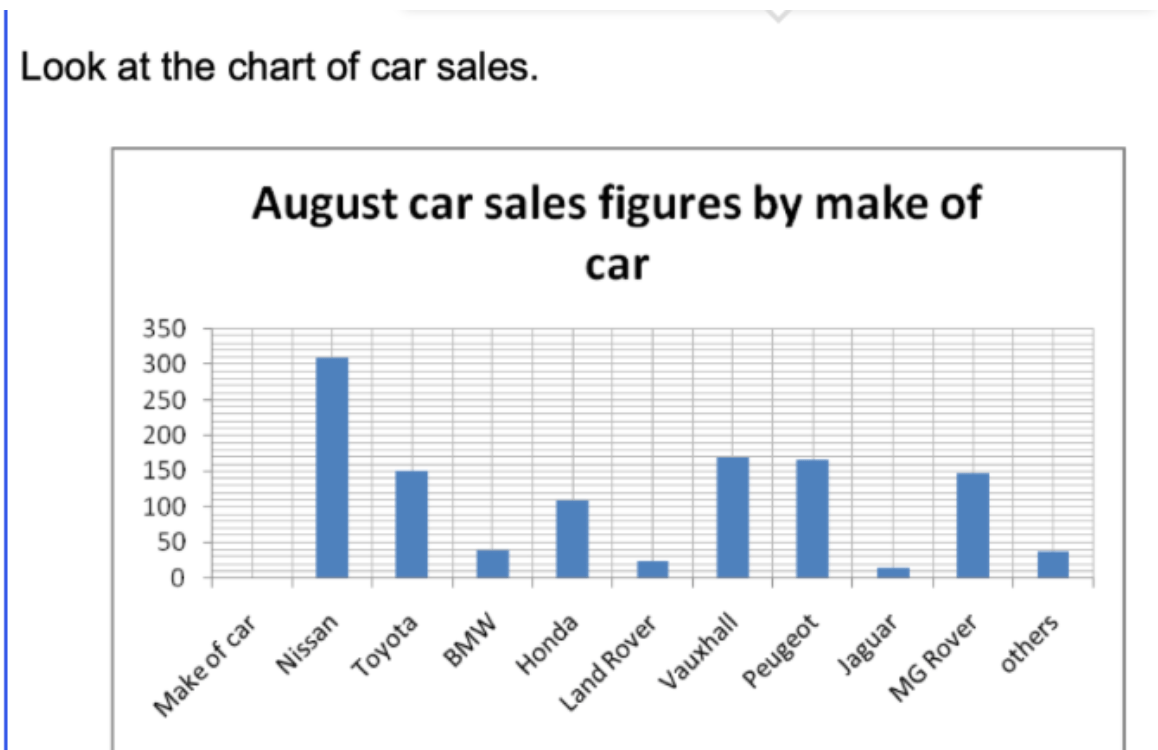
What is the average car sales
about 100-115
_________is the term used to explain how hot or cold an object is.
Temperature
Temperature is defined as the average potential energy of particles in a substance.
True or False
False
Put into scientific notation 0.04 kg
4.0x10^-2 kg
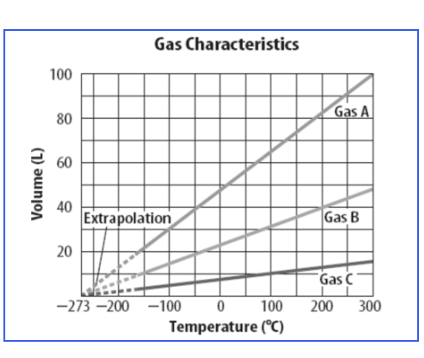
Look at the graph. What is the difference in volume between the Gas A and C at 300 degrees?
about 82 Liters
Ice is an example of matter in the ___ state.
solid
_____ consists of positively and negatively charged particles.
Plasma
Give the number of significant figures 11.0006g
6
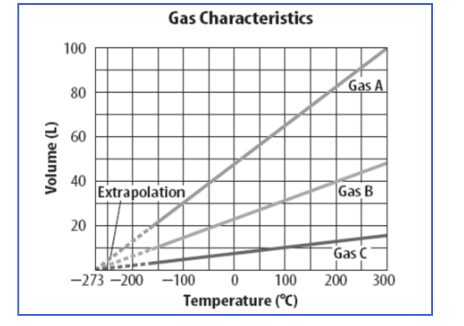
Which gas increased the most in volume with temperature increasing?
Gas A
The temperature at which a solid begins to liquefy is its point.
melting
Give a example of a plasma state of matter
answer could be:
stars
lighting
Put into scientific notation 150
1.5x10^2
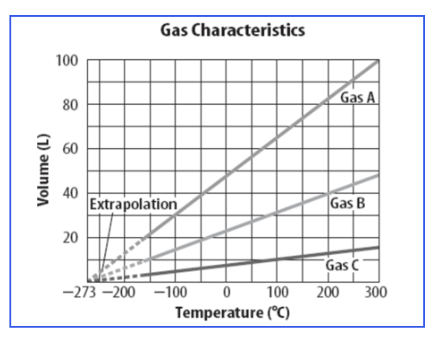
At approximately what temperature is the volume of gas A about 30 Liters?
-100
The amount of energy required to change a substance from the solid phase to the liquid phase is known as the ________ .
Heat of Fusion
In which state of matter are the particles likely to be closest together?
solid