The portion of a solution that dissolved another substance.
What is a solvent?
Name the three parts of an atom and give their charges.
Protons have a positive charge.
Electrons have a negative charge.
Neutrons have no charge.
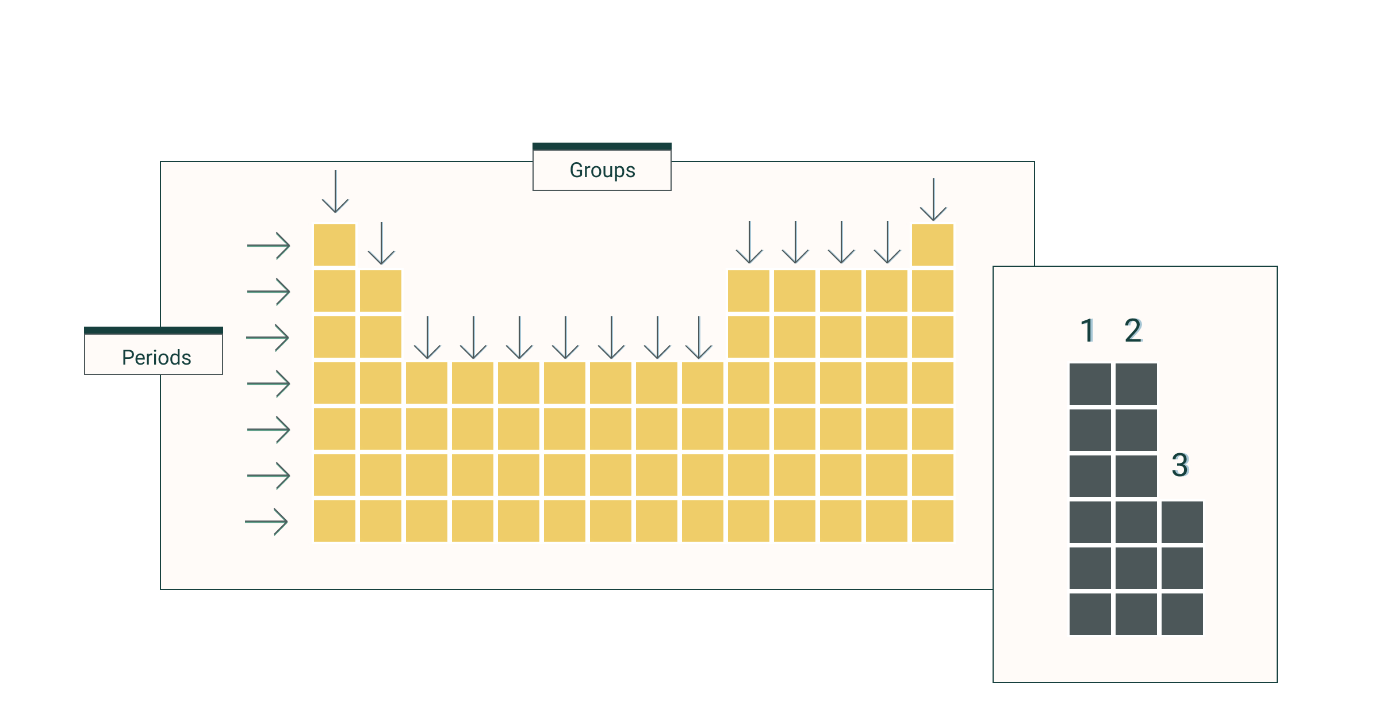
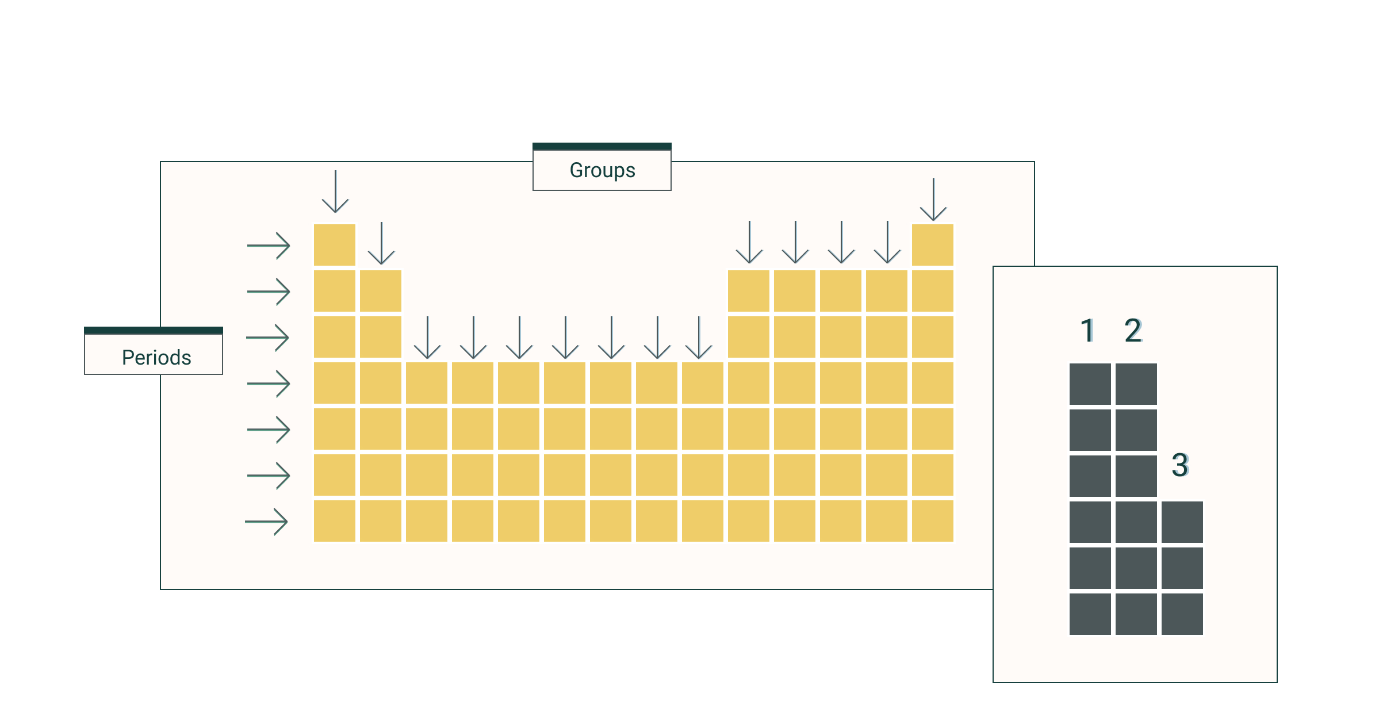
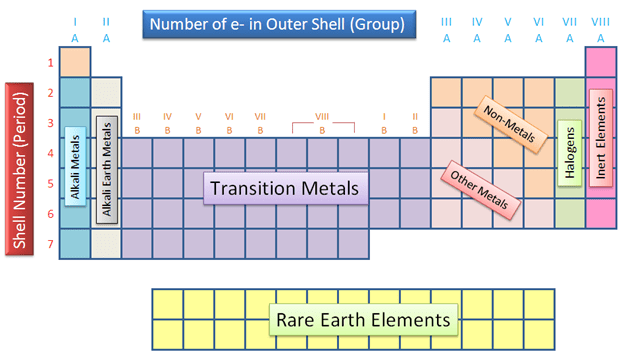
What do we call the rows on the periodic table?
Periods
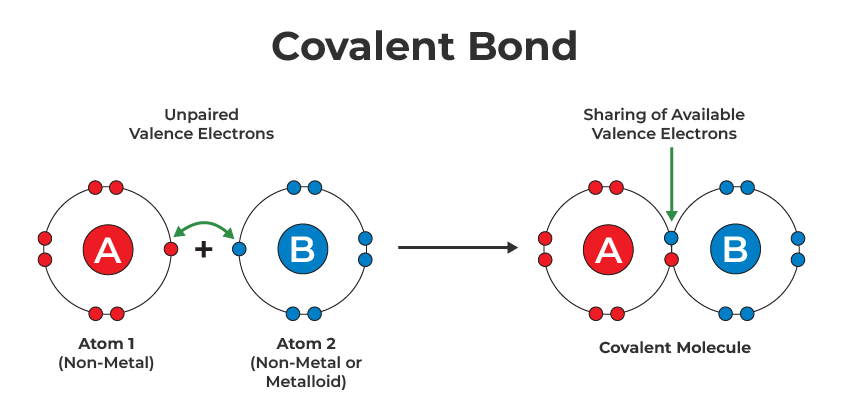
What kind of bonds form when nonmetals share valence electrons?
covalent
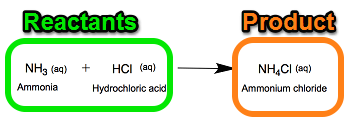
The name of the objects that result in Products being formed from a chemical reaction.
What are Reactants?
The portion of a solution that gets dissolved.
What is a solute?
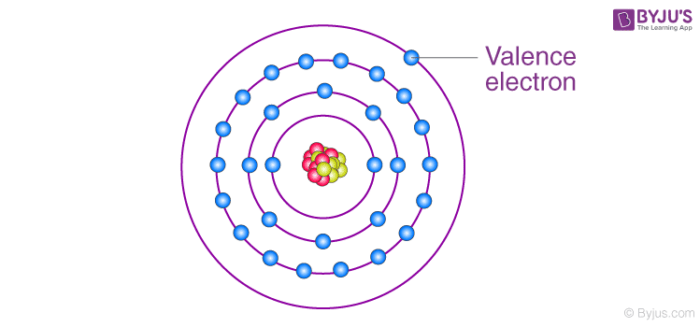
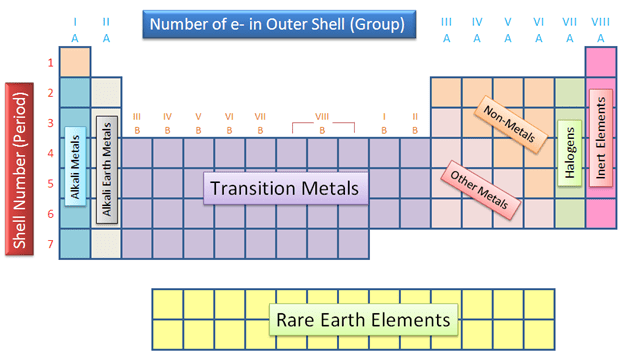
What do we call the electrons in the outermost energy shell?
Valence electrons
What do we call the columns on the periodic table?
Groups or Families
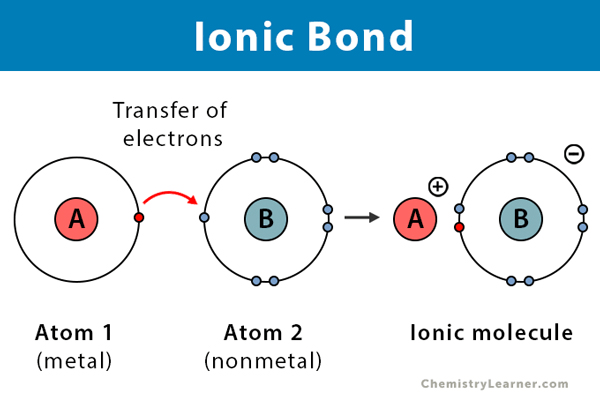
What kind of bonds form when a metal transfers valence electrons to a nonmetal?
ionic
What is a decomposition reaction?
The amount of salt in 500g of a 2.5% solution of salt water.
What is 12.5g of salt?
What must happen for an electron to jump to a different energy level?
It must lose or gain energy.
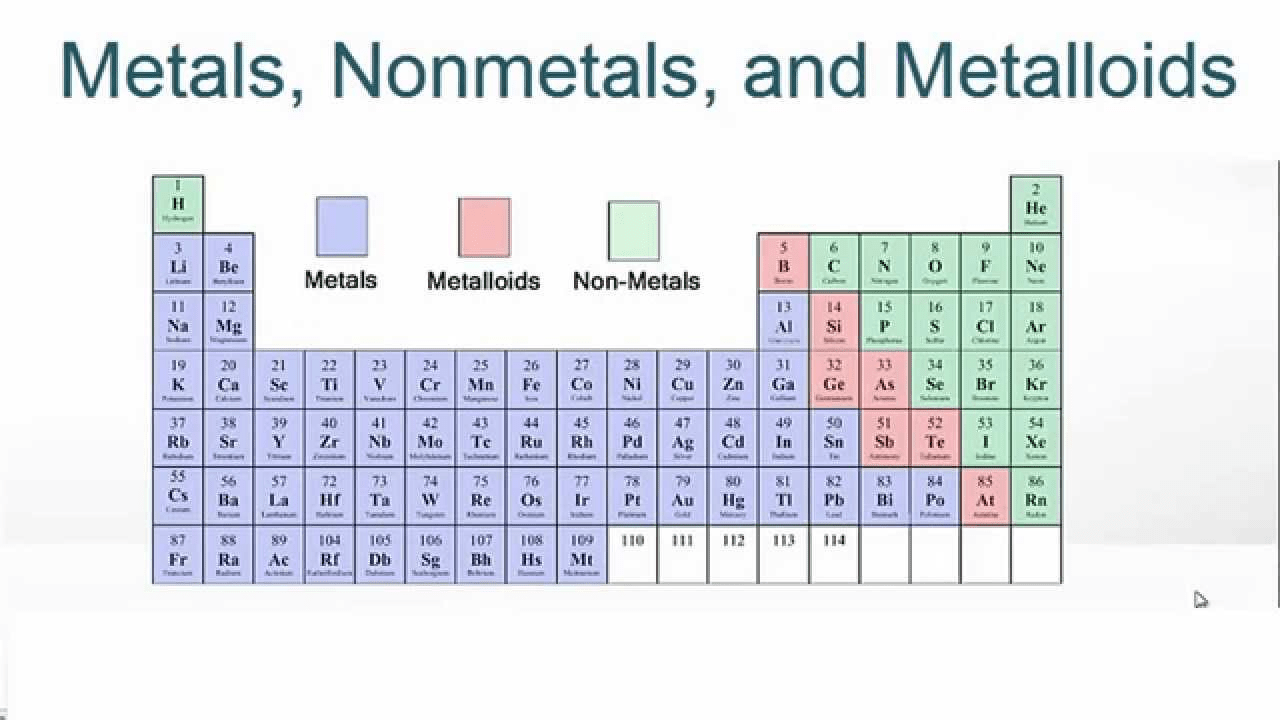
What are the three categories of elements on the periodic table?
The three major categories on the periodic table are the metals, nonmetals and metalloids.
What kind of bonds are forces of attraction between positive metal ions and the valence electrons that surround them
metallic bonds
The correct chemical formula of Lithium Fluoride. (E.g. not Li3F2)
LiF
What is 23%
The electron configuration for Carbon C(6).
(Level 1 = ?, Level 2 = ?, etc.)
Level 1 = 2
Level 2 = 8
What are the first two groups on the periodic table called?
Alkali and alkaline earth metals
The Electron Dot Structure for Flourine.
(See Whiteboard)
The name of the compound that forms after the following two chemicals react: Mg and OH−
What is Magnesium Hydroxide?
The name of the type of solution that forms when NaCl dissolves into H2O.
What is an amalgam?
The electron configuration for Zn (30).
(Level 1 = ?, Level 2 = ?, etc.)
Level 1 = 2
Level 2 = 8
Level 3 = 18
Level 4 = 2
What are the last two groups on the periodic table?
Halogens and noble gases
The Electron Dot Structure for Lithium.
(See Whiteboard)
The correct chemical formula when Copper(II) reacts with Hydroxide to form Copper Hydroxide.