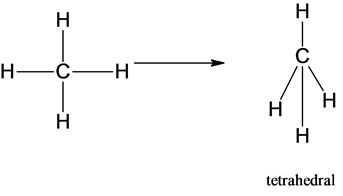Using what property of a molecule, is polarity determined?
Electronegativity (EN)
What is polarity?
Polarity is the quality of a molecule to be negatively or positively charged. This creates different types of bonds. And as we've always heard, opposites attract.
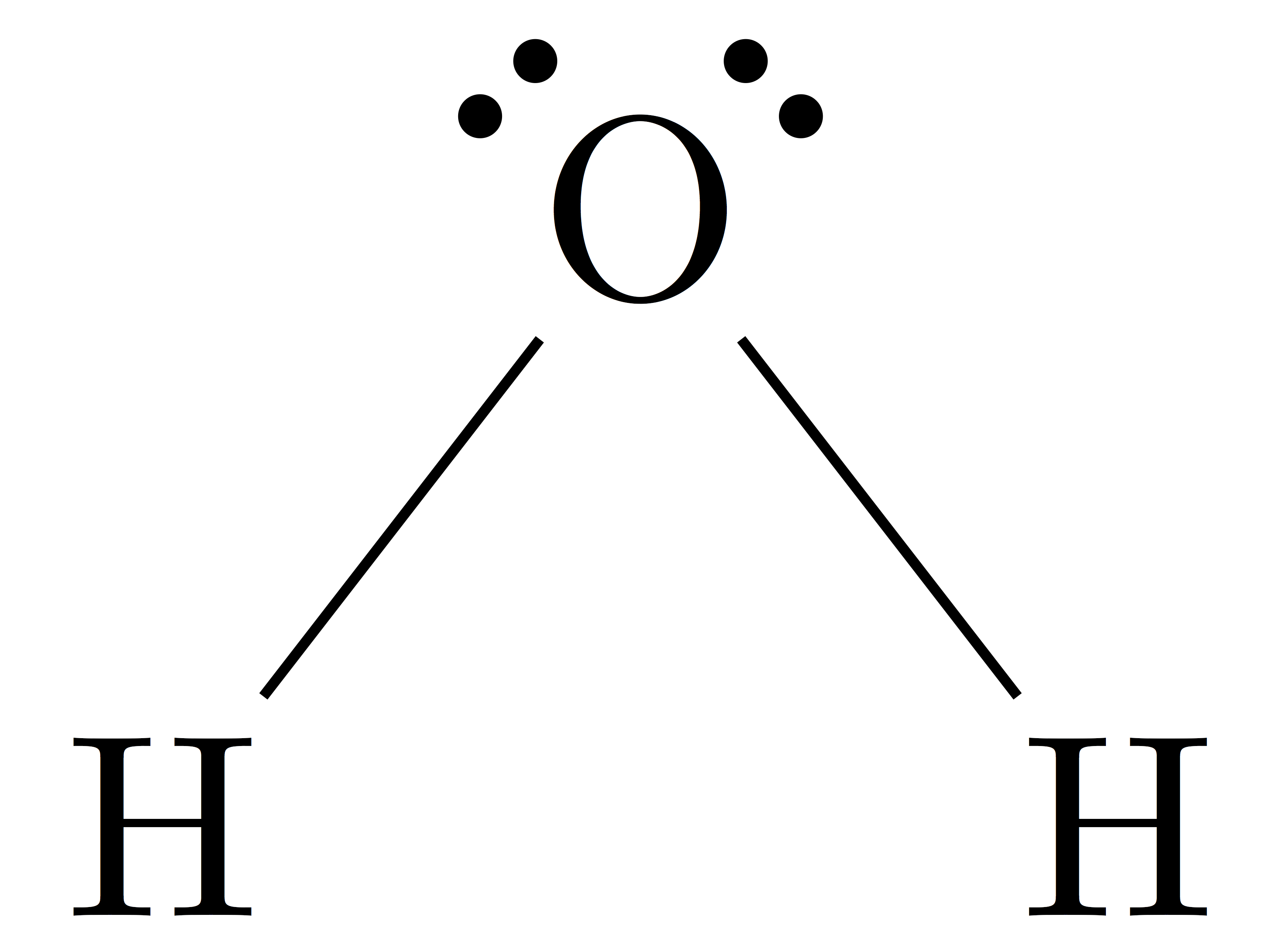
What is the geometry of the molecule H2O?
Bent
What level of Electronegativity is considered
Nonpolar Covalent?
What's an example?
An EN of 0-0.4
O2, I2, N2, (ect)
Difference between Intra And Inter Molecular forces?
Intra = Within species (Bonds represented with Solid lines.
Inter = Between species (Bonds represented by slashed line)

What is the shape of Carbon Dioxide CO2 molecule?
Linear
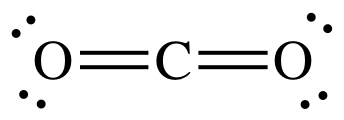
What levels of Electronegativity is considered Mod polar?
Give an example
0.5 - 0.9
N2O (It could be any other Mod polar)
What are the Intra molecular forces and the inter molecular?
Hydrogen bond, Ionic, Dipole interaction, covalent, London dispersion.
Intra -Ionic and Covalent binding
Inter - Hydrogen bond, Dipole interaction, London dispersion.
What is the shape of CH2O (Formaldehyde)
Trigonal planar

What level of electronegativity is considered Very polar?
Give an example?
1.0-1.9
H2O (any other very polar molecule)
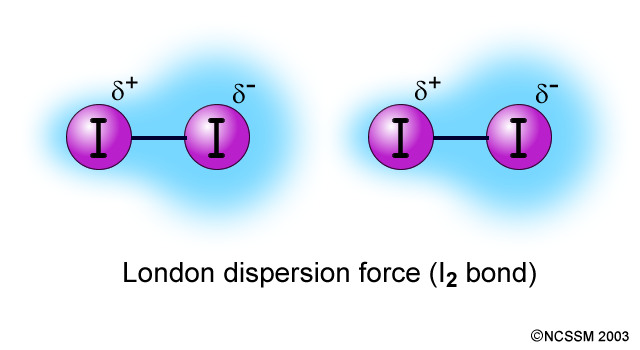
Define London dispersion.
Temporary dipoles are created in the electron cloud of the molecule because of electron movement. These attractions are constantly broken and reformed.
What is the shape of NH3 (Ammonia)
Trigonal Pyramid

What is a molecule considered when it has an electronegativity higher than 1.9?
Give an example
Ionic
NaCl
Define Dipole-dipole interaction.
The attraction between permanent Positive and Negative poles that are created by the partial positive and negative charges.

What is the shape of CH4 (methane)?
Tetrahedral