What is the smallest unit of matter that makes up an element?
Atom.
What is an element?
A substance that cannot be broken down into simpler chemical substances.
What are the two main types of chemical bonds?
Ionic and covalent bonds.
What property of water allows it to stick to itself?
Cohesion.
What does pH stand for?
Potential Hydrogen
Name the three subatomic particles and their charges.
Proton (+), Neutron (neutral), Electron (-).
What are isotopes? Give an example.
Isotopes are atoms of the same element with different numbers of neutrons, e.g., Carbon-12 and Carbon-14.
Balance the following equation:


What kind of bond forms between water molecules?
Hydrogen bond.
What is the impact of ocean acidification?
It reduces the pH of the ocean, harming marine life.
What determines the atomic number of an element?
The number of protons.
How many protons and neutrons does Carbon-14 have?
6 protons and 8 neutrons.
What is a covalent bond?
A bond formed when two atoms share electrons.
What is a solution?
A mixture where one substance (solute) is dissolved in another (solvent)
If a solution containing 1x10-3 H+ ions, what is the pH of the solution?
pH=3
Where are protons and neutrons located in an atom?
In the nucleus.
How are isotopes used in science?
They are used in radiometric dating and cancer treatment.
What is an Ionic bond?
When an atom steals a valence electron from another atom
What is capillary action, and how does it relate to water?
The ability of water to move up narrow tubes, like in plant roots, due to cohesion and adhesion.
What is the color change of litmus paper in an acidic solution?
Red
What is the difference between atomic number and atomic mass?
Atomic number is the number of protons; atomic mass is the total of protons and neutrons.
Sulfur has is listed as having an atomic mass of 32.065. What is the most common isotope of sulfur on Earth?
Sulfur-32
What type of bond forms between the hydrogen of one water molecule and the and oxygen of another water molecule?
A hydrogen bond
What is the difference between a solvent and a solute?
The solvent dissolves the solute.
What is the typical pH range that pH paper can test?
From 0 to 14.
What is the charge of an atom that loses an electron?
+1 charge
Carbon-14 and Carbon-12 are both isotopes of carbon. Assuming they are neutrally charged, how many more electrons does carbon-14 have than carbon-12?
0
They are neutrally charged so that means they both have 6 electrons (because carbon always has 6 protons).
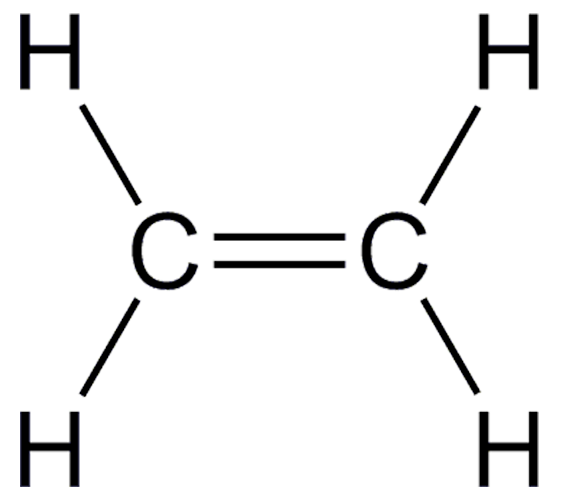
What kind of bond forms between the 2 carbon atoms?
How many electrons are being shared between the 2 carbons?
How many single bonds are there?
Write the molecular formula.
Double bond between the carbons.
four electrons are being shared.
four single bonds.
C2H4
How does water dissolve ionic compounds like salt?
The positive side of water attracts the anions, and the negative side attracts the cations.
What happens to the pH when CO₂ dissolves in water, and how can indicators show this change?
The pH decreases, becoming more acidic, and indicators like bromothymol blue can change from blue to yellow, signaling increased acidity.