The lab equipment you would use to measure volume.
What is a graduated cylinder?
The number of significant figures in 4500 kg
What is 2?
The SI unit for mass.
What is the kilogram (kg)?
The number of feet in 37.5 inches.
What is 3.13 feet?
The difference between an independent and dependent variable.
Independent- what you changed
Dependent- what you measured based on that change
A target showing the following scenarios:
- Both accurate and precise
- Accurate but not precise
(See drawings)
58000 g in scientific notation
What is 5.8 x 104 g?
The number of particles in a mole.
What is 6.02 x 1023?
The number of centimeters in 19.82 km.
What is 1982000 cm? (1.982 x 106 cm)
The reason for only having one independent variable.
What is: so that you know exactly what is causing the results that you are measuring?
Percent error is an indication of the _________ of a measurement.
What is accuracy?
9.2203 x 10-8 s in standard notation
What is 0.000000092203 s?
250 mg = _________ g
0.25 g
The number of molecules in 9.2 x 10-2 moles of water.
What is 5.5 x 1022 molecules of water?
The importance of control variables.
What is: to ensure that you only have one independent variable at a time?
The length of the green rectangle.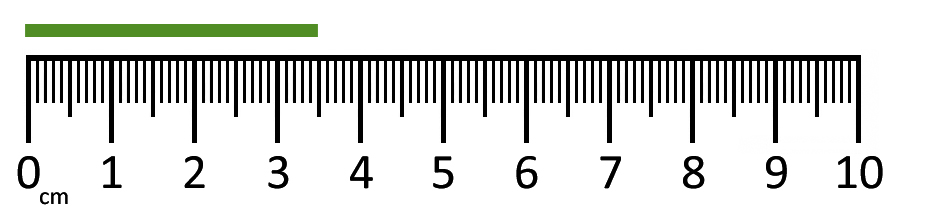
What is 3.48 cm?
The answer to the following with the correct number of sig figs:
2.33 m + 5.7 m - 1.002 m + 344 m
What is 351 m?
9.8 Mmol = _______ mmol
9800000000 mmol
The number of hours in 12345 seconds.
What is 3.4292 hours?
All the steps of the scientific method.
1. Make an observation
2. Ask a question
3. Do background research
4. Make a hypothesis
5. Test your hypothesis
6. Analyze your data and draw a conclusion
7. Communicate your results
The % error of your measurement if you measured a volume of 59.6 mL and found out the accepted value was 65.0 mL.
What is 8.31% error?
(3.78 x 10-6 g) / (9.2 x 10-2 mol)
4.1 x 10-5 g/mol
The mass of an object that has a density of 2.7 g/mL and a volume of 100.0 mL.
What is 270 g?
The number of millimeters in 2.5 yds. (1 in = 2.54 cm)
What is 2300 mm?
Identify the independent, dependent, and control variables in the following scenario:
Different types of rose bushes are grown in a greenhouse for two months. The number of flowers on each bush is counted at the end of the experiment.
Independent: type of rose bush
Dependent: number of flowers
Controls: light, soil, water, temperature, humidity, time, initial number of flowers, etc...