Electrons have a ________ Charge.
Negative
A chemical reaction with other elements
Reactivity
This section of the periodic table has vertical columns top to bottom of elements or "family."
Groups
The number of protons in the nucleus of an atom; the atomic number is the same for all atoms of an element.
Atomic Number
What has a high electrical conductivity
high luster (shiny looking)
malleable (can deform, stretching, bending)
Metal
Neutrons are found where in an atom?
The center
What is it called when a certain type of metal is a good conductor?
Conductivity
This section of the periodic table has horizontal row from left to right of elements. Helps finds the energy level of an element.
Periods
A region around the nucleus of an atom where electrons are likely to be found.
Electron Cloud
Which group of elements are in the upper-right coroner of the periodic table
Not lustrous (not shiny)
Poor conductors of electricity
Non-metals
This is where you will find Protons and Neutrons
The Nucleus
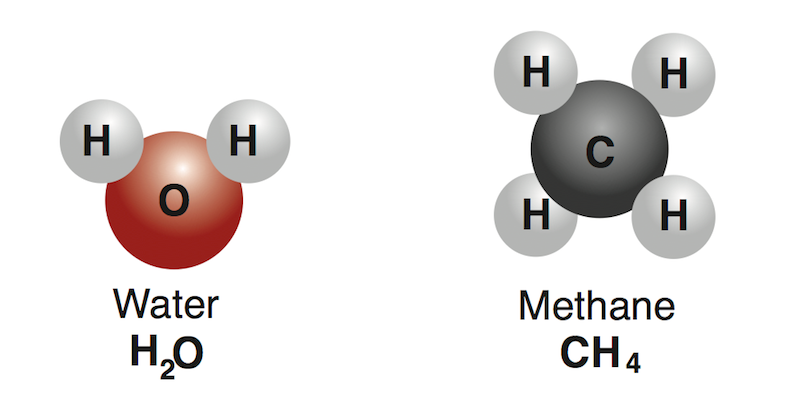
This a example of a
Compound
What are the three groupings of the Periodic Table?
Metals, Non-metals, Metalloids
The sum of the numbers of protons and neutrons in the nucleus of an atom.
Mass Number
Alkali Metals are _______solids
soft
A particle smaller than an atom
Subatomic
This is an example of an
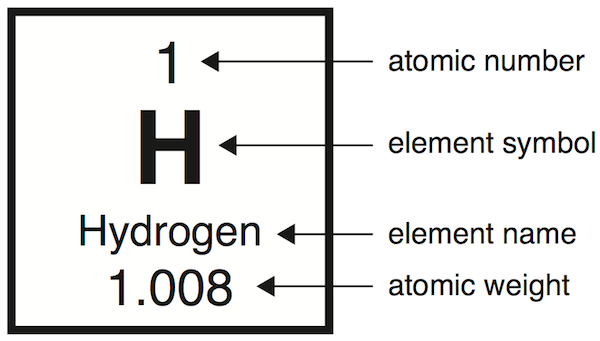
Element
Where are the non-metals located on the periodic table?
Upper-right corner
A subatomic particle that has a positive charge
Proton
Alkaline Earth Metals are found in the_____
earth
This is the smallest part of matter
Atom
This is an example of a
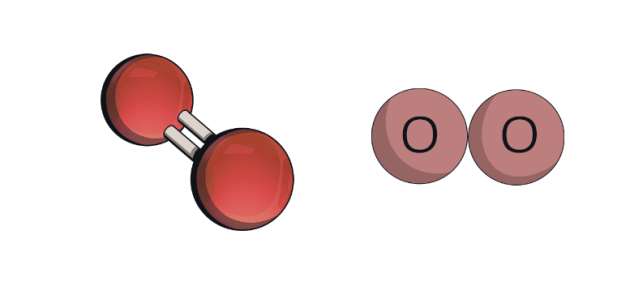
Molecule
Where are the Metalloids located?
On the stair-step line
The smallest unit of element that maintains the properties of that element.
Atom
Give one example of a transition metal
Answers may vary