a pure substance that cannot be broken down chemically into simpler substances
What is an element?
easily broken
What is brittle?
DAILY DOUBLE!!!
This is what will happen to an object with a density of 1.33 g/cm cubed in a beaker of tap water when it is dropped in it
A. The object will sink
B. The object will float
What is A.?
This is the physical representation of all 118+ known elements in the entire universe (hint: they keep adding to it all of the time!)
What is the Periodic Table?
For example, the density of a certain liquid is 0.75 g/mL. Tell me if it will sink or float in water.
What is float?
a substance that is not mixed with any other substance
What is a pure substance?
the amount of matter that will fit into a given amount of space
What is density?
This is the formula to find the density of an object (density=?)
What is density=mass divided by volume?
This is the chemical symbol for Nitrogen
What is N?
This is the formula needed to find the volume of a regular cube
What is length times width times height?
change which alters the physical properties of a substance without changing its identity
What is physical change?
A material that does not allow heat or electrons to move through it easily.
What is insulator?
This is the unit needed on the end of a number for the volume of a solid
A. mL
B. cm3
C. L
D. kg
What is cm3?
This is the chemical symbol for Chlorine
What is Cl?
DAILY DOUBLE!!!
Which of the following lists of properties best describes nonmetals?
A. Brittle, shiny, conductor
B. Malleable, shiny, conductor
C. Brittle, dull, insulator
D. Malleable, dull, insulator
What is C.?
DAILY DOUBLE!!!
Characteristic that cannot be observed without altering the substance
What are chemical properties?
how the surface of a mineral appears when it reflects light (e.g., shiny or dull)
What is luster?
DAILY DOUBLE!!!
This is the method you use to find the volume of an irregularly shaped solid (explain how you find it using a graduated cylinder)
What is displacement method, final volume minus initial volume?
Two liquids are mixed together. Which of the following does NOT identify the formation of a new substance?
A. A gas is produced.
B. One liquid dissolves into the other.
C. The temperature changes.
D. A solid is formed.
What is B.?


Which of the following would be found only in the Compounds side of the Venn diagram shown above?
What is A. II and III?
the number written to the right and slightly below an element in a chemical formula; represents the number of atoms of an element present in a chemical formula
What is a subscript?
A solid inorganic substance of natural occurrence
What is mineral?
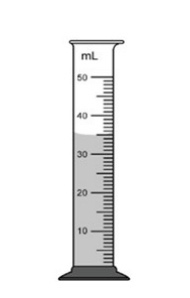
The volume of an unknown liquid is shown in the graduated cylinder. The mass of the liquid is 105 grams. Tell me the density of the unknown liquid, plus the correct unit that needs to be used with it.
What is 3 g/mL?
Students were investigating two clear solutions. Students added 2 mL of one solution to a beaker of the other solution. The result is pictured below.

A student observed that the temperature of the beaker did not have any noticeable changes, however the mass of the substances in the beaker increased. A red precipitate formed where the substance was poured into the beaker. The precipitate separated from the solution and fell to the bottom of the beaker. Which observation provides the best evidence that a chemical change formed a new substance in the beaker?
A. A precipitate is formed.
B. The precipitate fell to the bottom.
C. The temperature did not change.
D. There is an increase in mass.
What is B.?
A student performs an investigation on a 5 g sample of an element. The student recorded observations below.

Based on the physical properties observed, the student should classify this sample as a a/an —
A. compound
B. nonmetal
C. metal
D. metalloid
What is D.?
a representation of a compound in which the elements are represented by their symbols and subscripts represent the number of atoms of each element
What is a chemical formula?
Distance and direction of an object's change in position from the starting point
What is displacement?
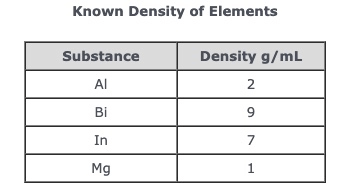 A student performs an investigation to determine the density of an unknown sample. The student drops the 10 g sample into a graduated cylinder with 30 mL of water. The water rises to 35 mL. Based on the chart and information above, what is the most likely identity of the sample?
A student performs an investigation to determine the density of an unknown sample. The student drops the 10 g sample into a graduated cylinder with 30 mL of water. The water rises to 35 mL. Based on the chart and information above, what is the most likely identity of the sample?
A. Aluminum
B. Bismuth
C. Indium
D. Magnesium
What is A.?
Phosphorus, Lithium, and Fluorine are elements that are found on the Periodic Table. Students conduct several tests involving samples of these three elements at normal room temperature.
Based on the physical properties of metals, nonmetals, and metalloids, which observation is correct?
A. Fluorine is malleable, and lithium conducts electricity.
B. Lithium is malleable and conducts electricity.
C. Phosphorus is malleable and conducts electricity.
D. All three elements are malleable and conduct electricity.
What is B.?

What is the density of the rock sample shown in the illustration above?
What is C.?