How many electrons does sulfur need to have a full valence shell if it is in Group 16?
2
two
An ionic bond will always form when these two types of elements react.
metal and a nonmetal
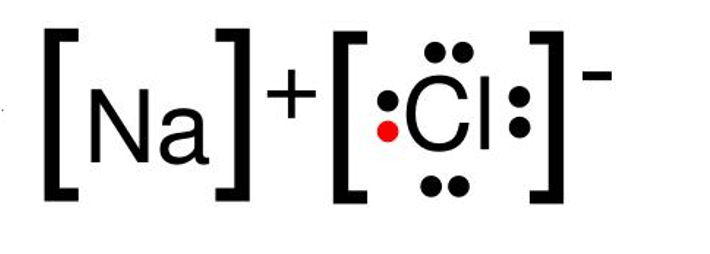
A covalent bond will always form when these two types of elements react.
nonmetal and nonmetal
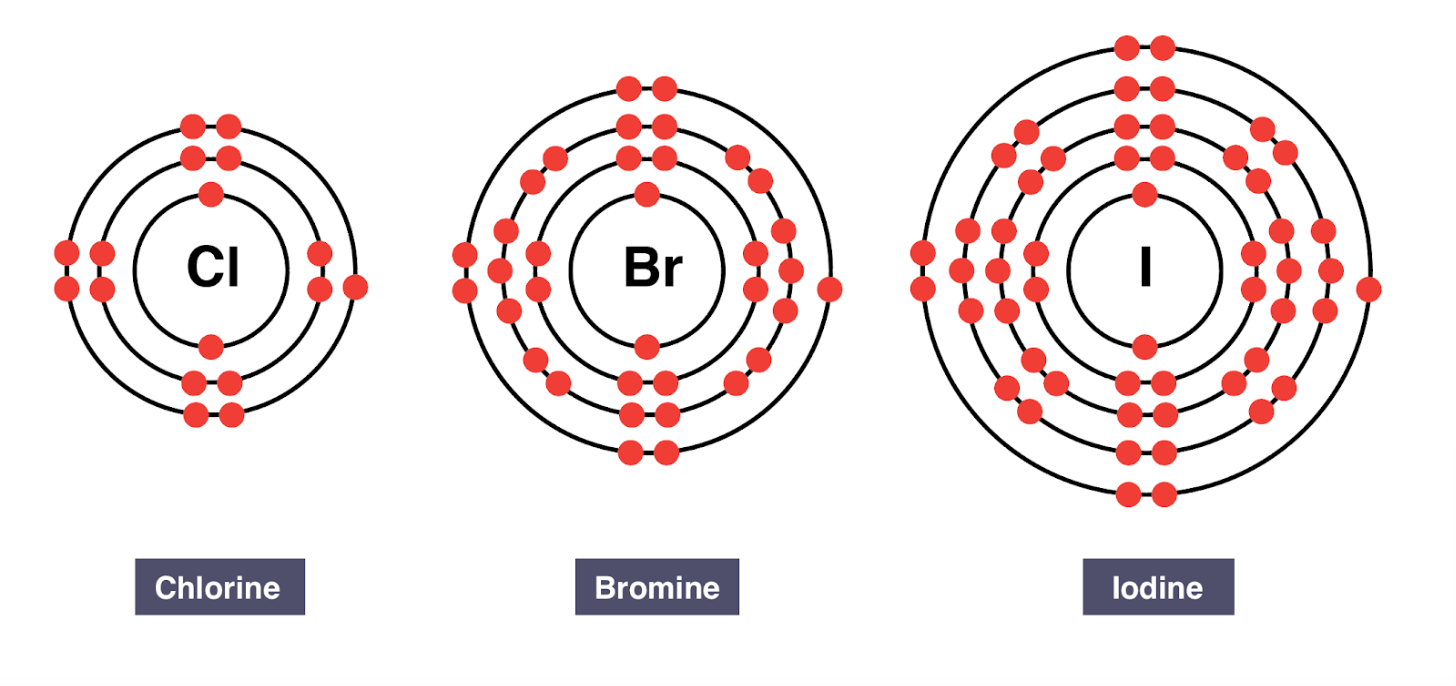
What do we call the electrons in the outermost energy level?
valence electrons
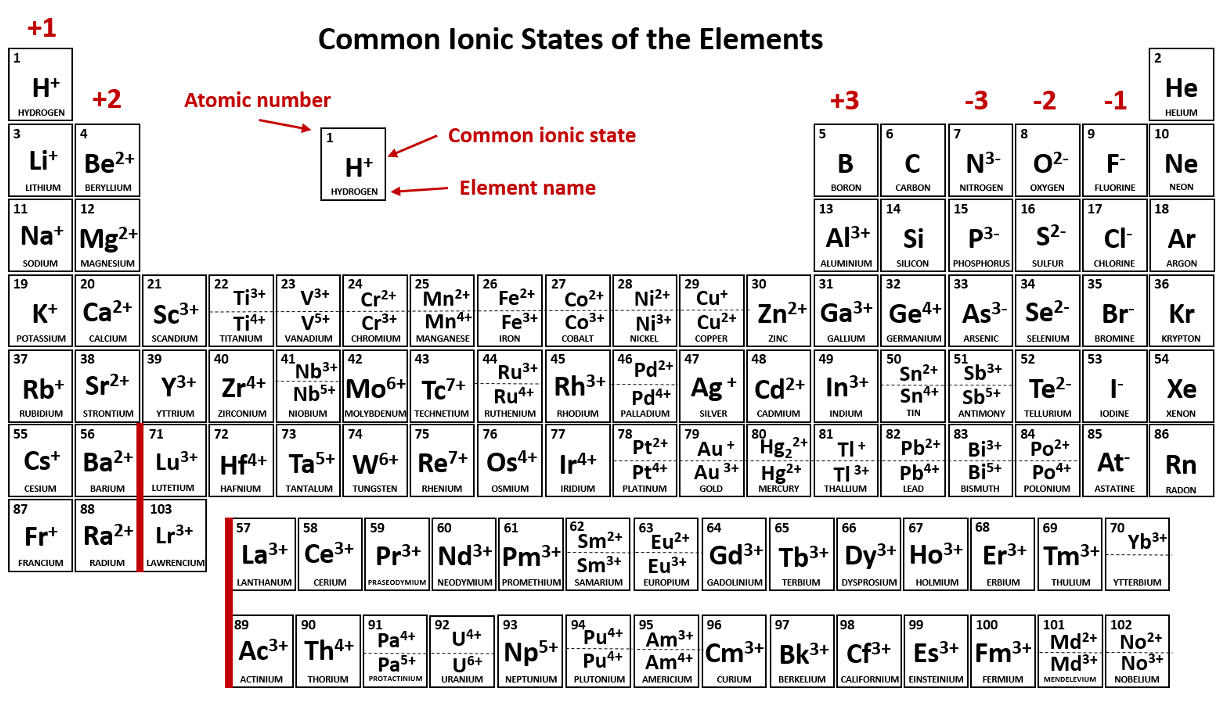
An atom or molecule that has a net negative or positive charge is called a/an _____.
ion
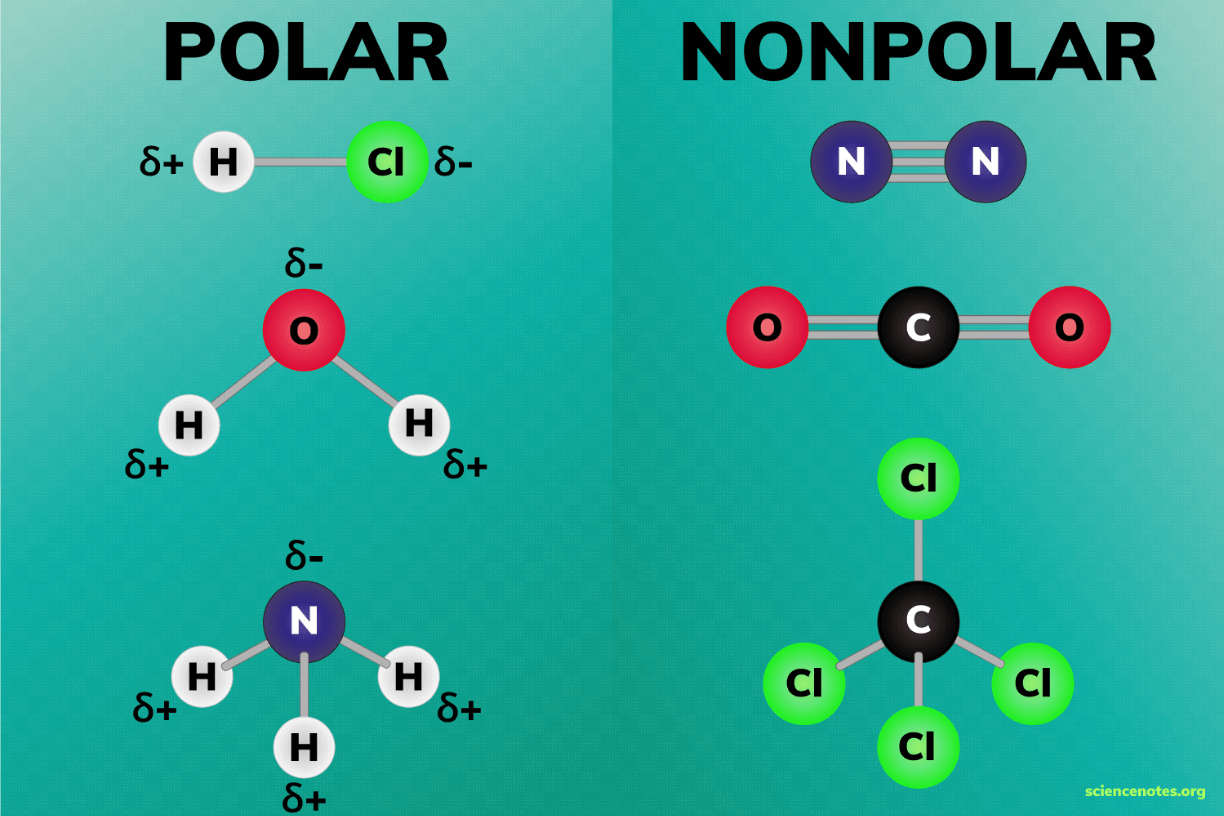
When an atom hogs/attracts electrons in a covalent bond and one side of the molecule is slightly negative and the other side is slightly positive, it is said to be _____.
Polar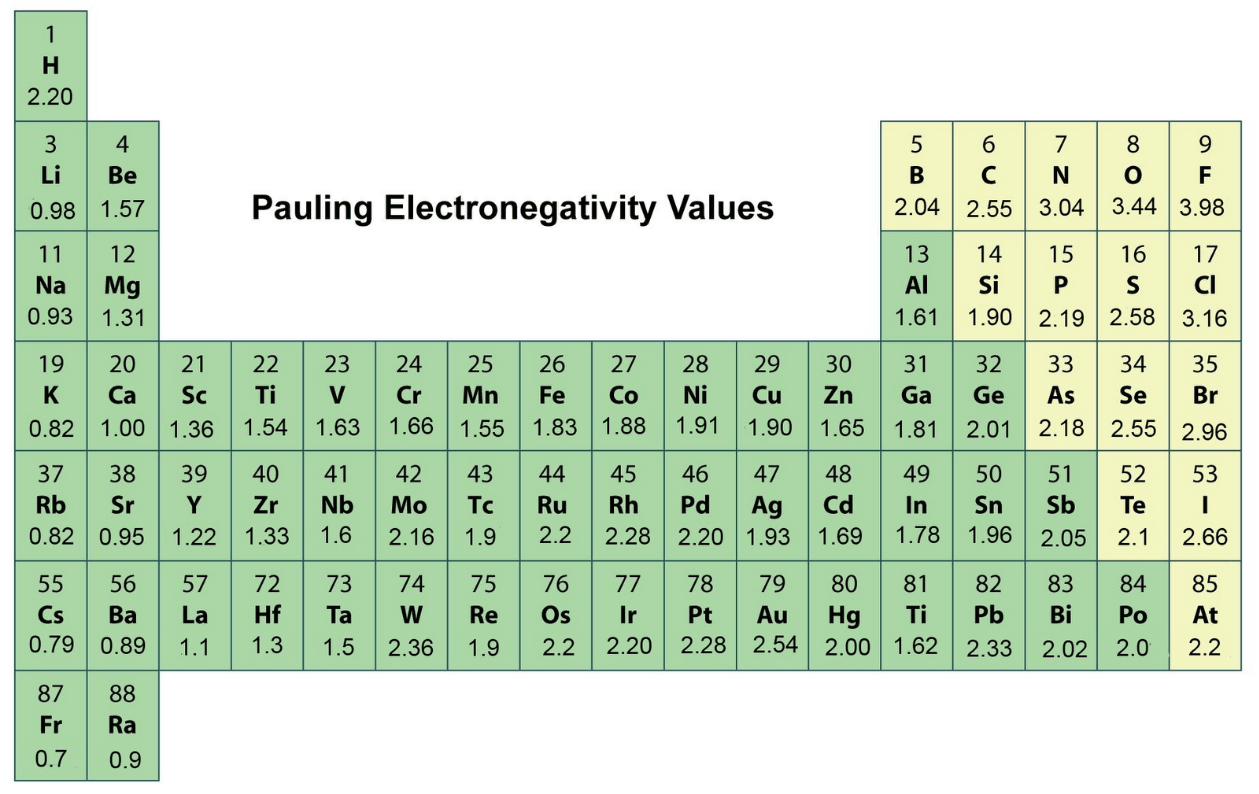
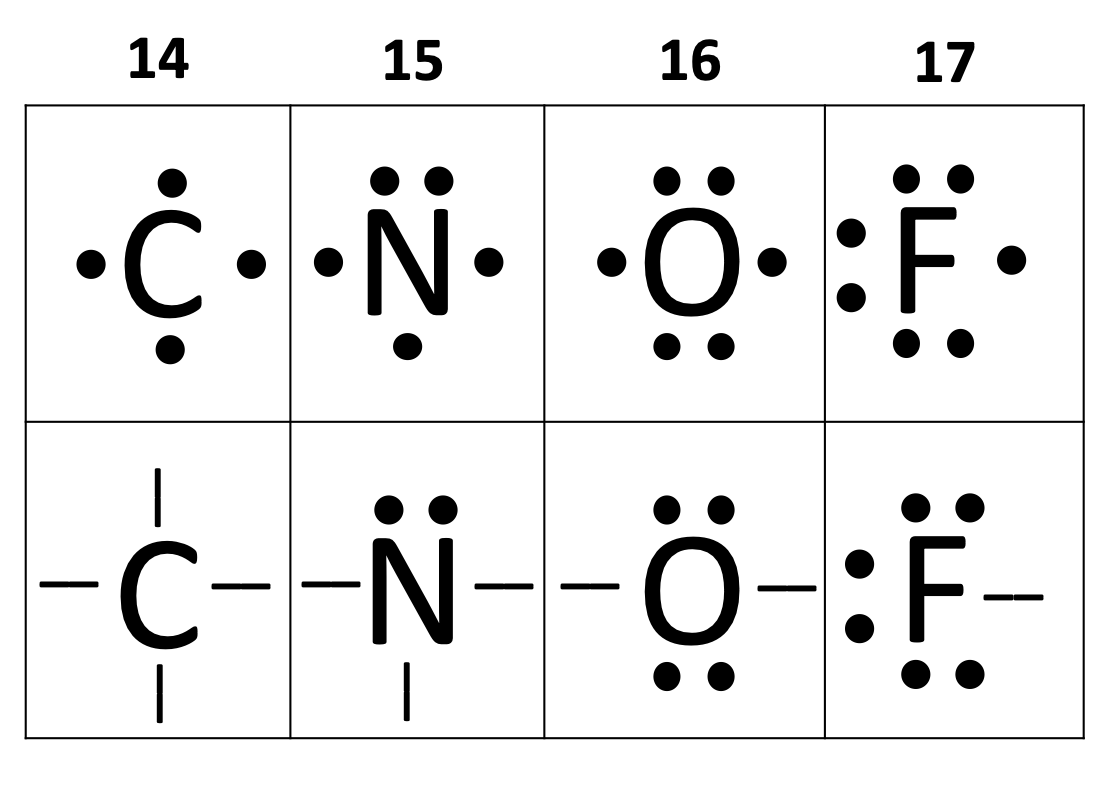
Elements require how many electrons in the outermost shell to be considered stable (except for the 1st energy level)?
8
eight
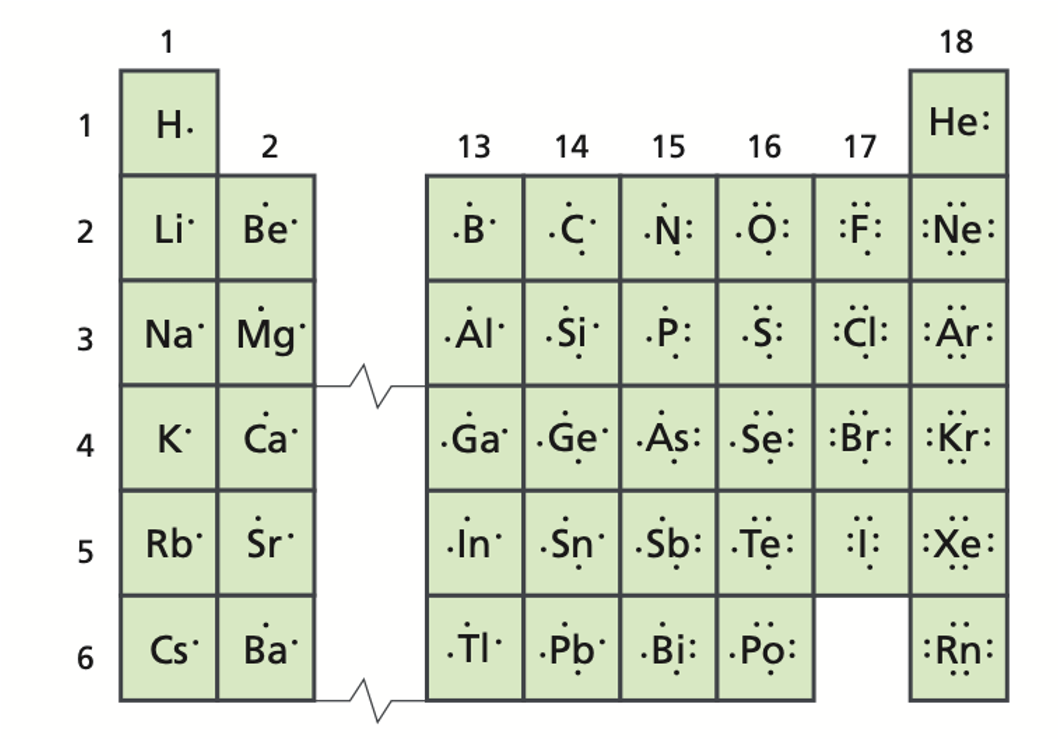
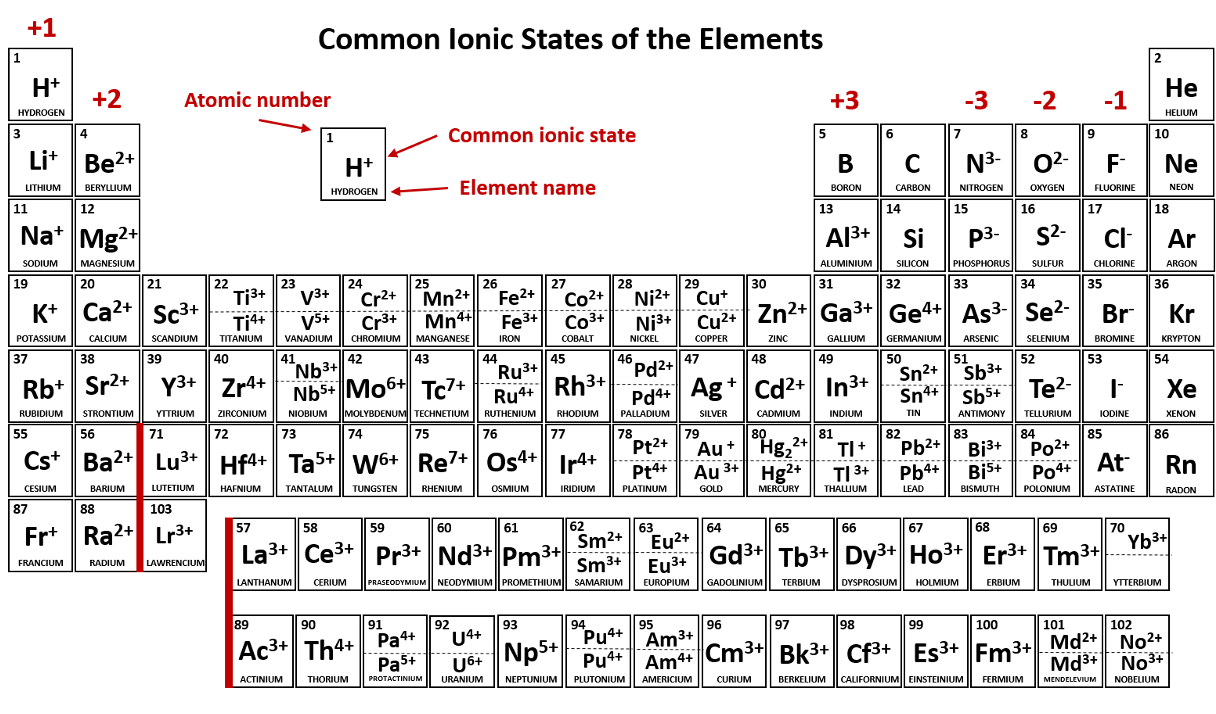
A positively charged ion is called a/an _____.
cation (Li+, Na+, Cu+, Cu2+, NH4+)
Will 2 noble gases want to bond with one another?
No
When two or more atoms share electrons it is called what type of bond?
covalent bond
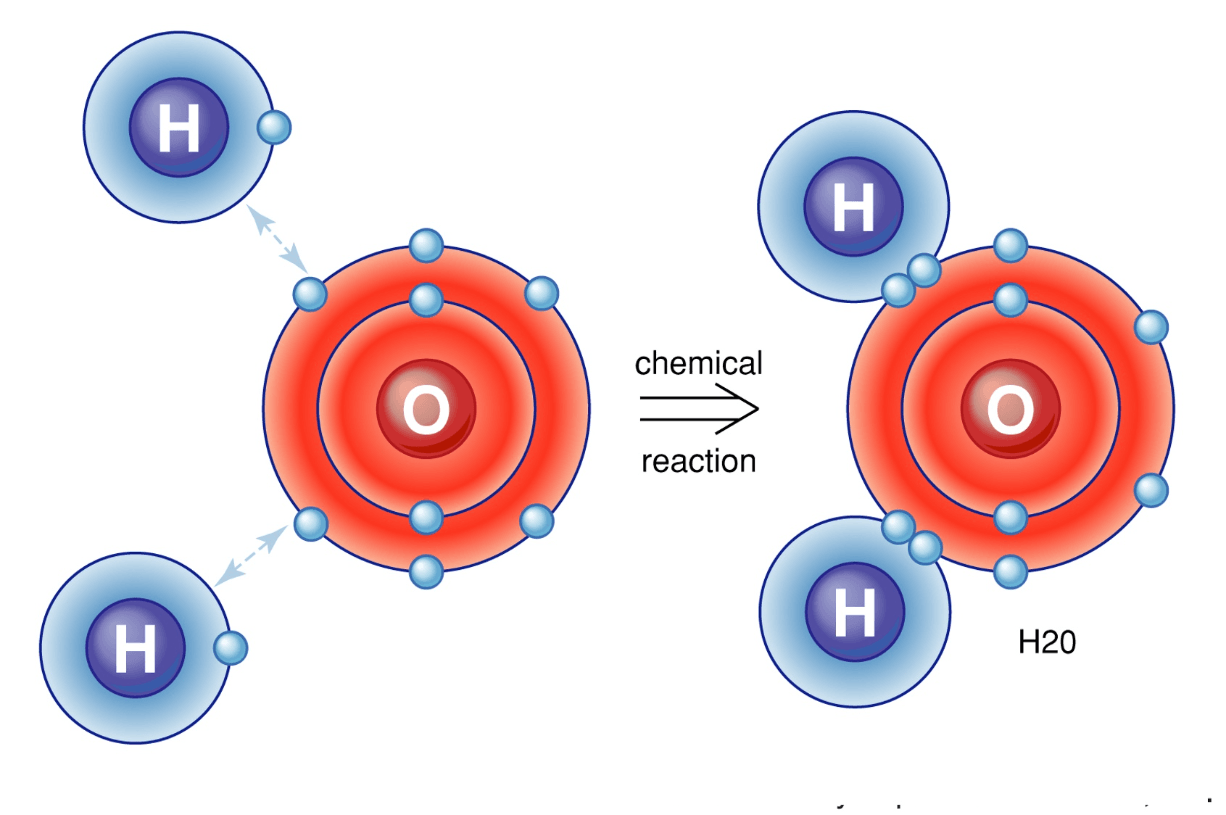
Alkali metals will always ionize to form ions with a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)
+1

Draw the Lewis dot structure between two Bromines showing their covalent bond.
If an atom loses an electron, then that atom will become ____ charged. (positively or negatively)
positively charged
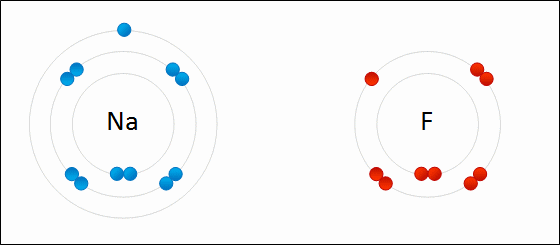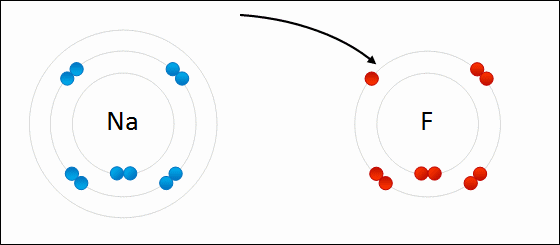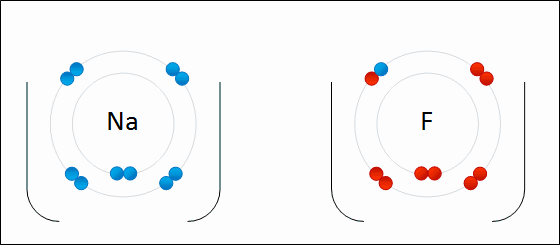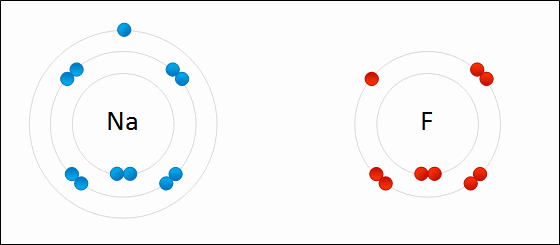
Draw Beryllium bonding with Nitrogen. Find the chemical formula.
Be3N2
Covalent molecules that have an uneven distribution of electrons are called _____.
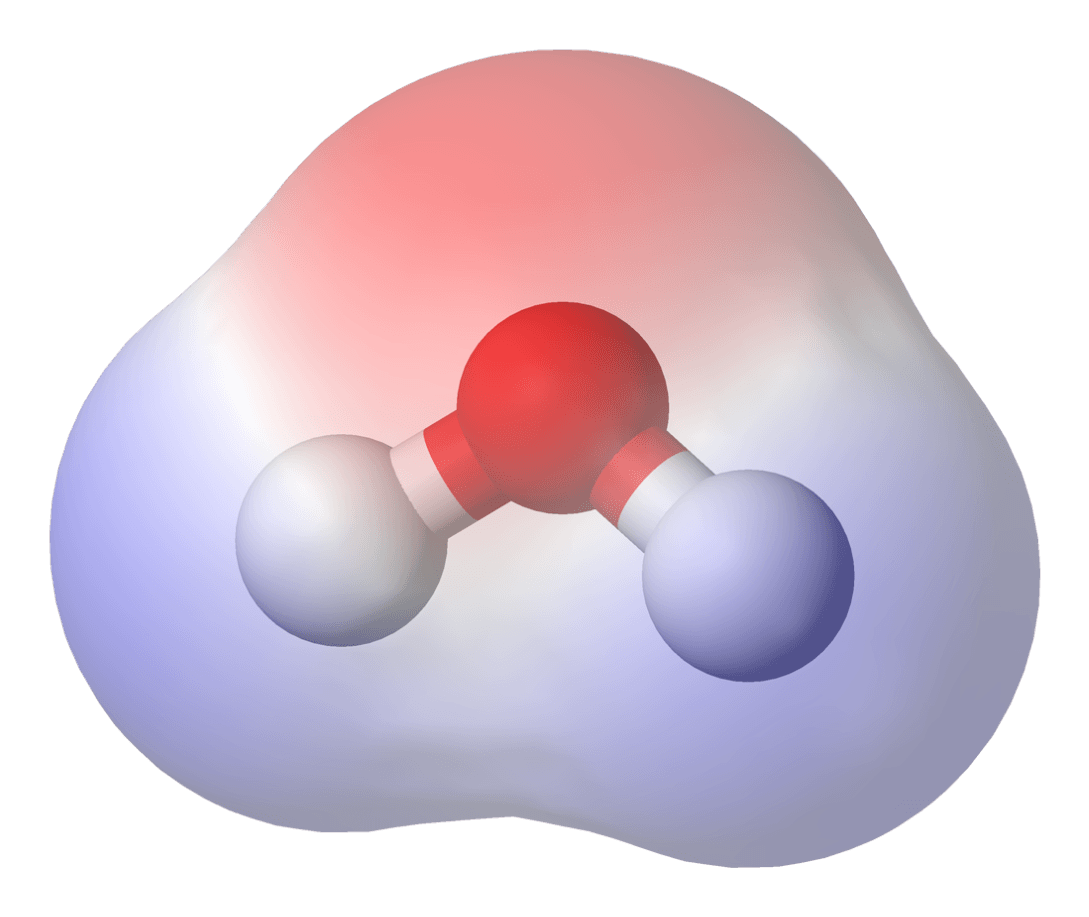
polar
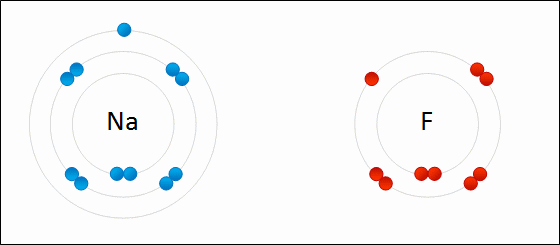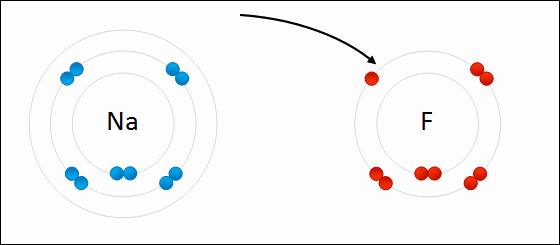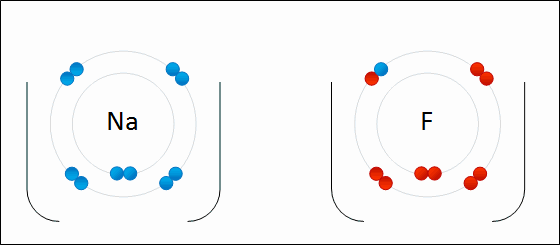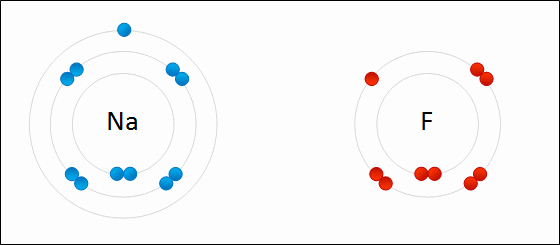
The type of bond formed when two or more atoms transfer electrons.
ionic bonding
A negatively charged ion is called a/an _____.
anion
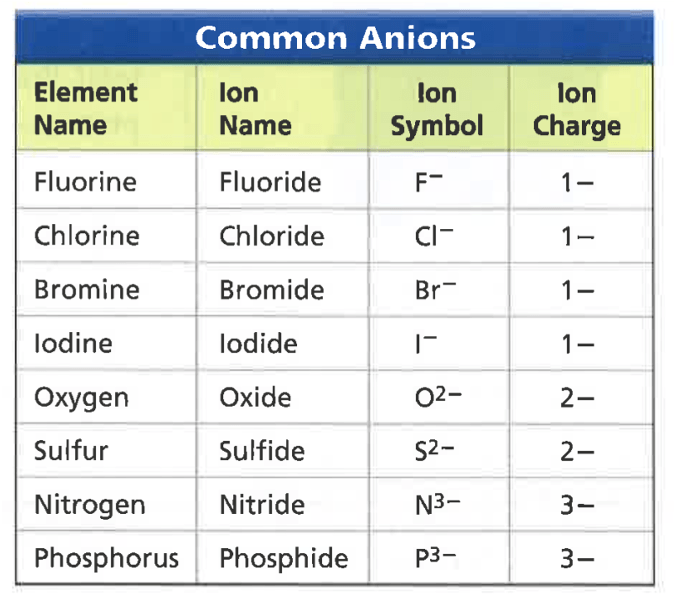
Combine Carbon and Flourine. What is the chemical formula?
CF4

If an atom gains an electron, then that atom will become ____ charged. (positively or negatively)
negatively charged

Metals in the Alkaline earth metal family always form ions that have a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)
+2
What is the name of N2O4?
Dinitrogen tetroxide
What is the name of FeO?
Iron (II) Oxide
Halogens always ionize to form ions with a ____ charge. (Give number and sign; e.g. 1-, 1+, 2+, etc.)
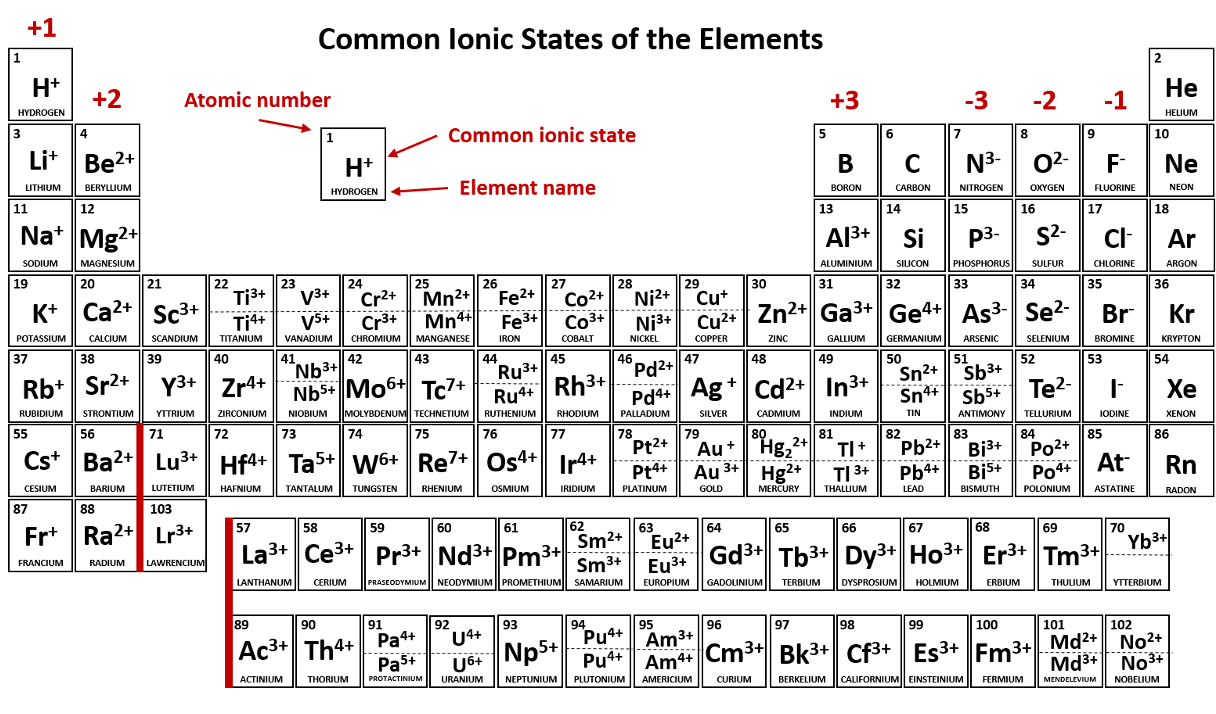
Write the chemical formula of Nitrogen Triflouride
NF3
What is the name of Li2SO4?
Lithium Sulfate
What charge does copper have in the ionic compound CuCl2?
copper(II)
How many bonds can carbon form? (i.e. How many valence electrons does carbon need)

Figure: charcoal is mostly pure carbon
4
four
What is the chemical formula for Lead (II) Hydroxide?
Pb(OH)2
What is the charge on iron (Fe) in the ionic compound Fe2O3?
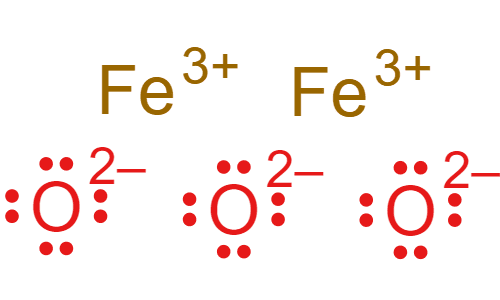
Double Jeopardy!
Atoms in covalent bonds don't have charges because the atoms are not losing or gaining any electrons.