 A simple compound whose molecules can join together to form polymers
A simple compound whose molecules can join together to form polymers
Monomer
A chemical compound containing the element carbon and usually the element hydrogen

Organic Compounds
A lipid composed of 3 fatty acids attached to a glycerol; most function as energy-storage molecules

Fats (Triglycerides)
An organic compound consisting mainly of carbon and hydrogen atoms linked by nonpolar covalent bonds, making the compound mostly hydrophobic. Includes fats, phospholipids, and steroids.

Lipids
A long molecule consisting of many similar or identical monomers linked together; makes up macromolecules

Polymer
A chemical group consisting of an oxygen atom bonded to a hydrogen atom. (OH)
Hydroxyl Group
Fatty acids that have the maximum number of hydrogen atoms possible and no double bonds; these solidify at room temperature

Saturated Fatty Acids
an organic compound composed only of the elements carbon and hydrogen

Hydrocarbons
organic molecules which combine to form living organisms; includes carbohydrates, lipids, proteins and nucleic acids
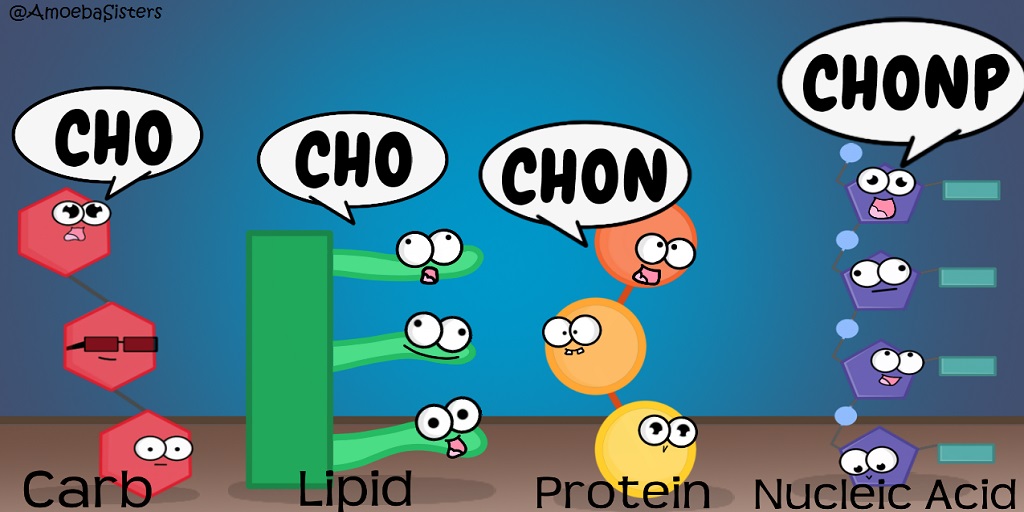
Biomolecules
The simplest carbohydrate; the monomers of disaccharides and polysaccharides

Monosaccharides
A fatty acid possessing one or more double bonds between the carbons in the hydrocarbon tail. Such bonding reduces the number of hydrogen atoms attached to the carbon skeleton; these do not solidify at room temperature

Unsaturated Fatty Acids
sugars (monosaccharides, disaccharides, and polysaccharides) that are broken down to glucose to provide energy.
Carbohydrates
A chemical reaction in which two molecules bond to each other with the removal of a water molecule.

Dehydration Synthesis
A sugar molecule consisting of two monosaccharides linked by a dehydration reaction.

Disaccharide
"water fearing"; pertaining to nonpolar molecules (or parts of molecules) that do not dissolve in water
Hydrophobic
Organic compounds with the same molecular formula but different structures and, therefore, different properties.

Isomers
Breaking down complex molecules by the chemical addition of water

Hydrolysis
Carbohydrates that are made up of more than two monosaccharides

Polysaccharides
"water-loving"; pertaining to polar or charged molecules (or parts of molecules) that are soluble in water
Hydrophilic
An unsaturated fat, formed artificially during hydrogenation of oils, containing one or more trans double bonds; linked to health risks; used to extend shelf life of processed foods

Trans Fats