The state of matter with particles that are close together and vibrate slowly in place.
What is a solid?
The equation and units for density
What is d = m ÷ v and g/cm3 or g/mL
Aluminum has a mass of 27 g and a volume of 10 cm3. Calculate the density.
What is 2.7 g/cm3
Density is constant for a substance and is often used to identify substances...intrinsic or extrinsic property?
What is intrinsic?
Ability to do work
What is energy?
State of matter where particles are close but move freely around each other
What is liquid?
One of these objects is the least dense.
What is C?
Carbon monoxide gas has a mass of 0.196 g and occupies a volume of 100 mL. Calculate the density.
What is 0.00196 g/mL ?
Which means amount of space...mass or volume?
What is volume?
amount of energy needed to raise 1 g of water by 1 degree Celsius
What is calorie?
Coldest theoretical temperature...[use a vocab word or a temperature value]
What is 0 K, -273 Celsius, -459 Fahrenheit?
OR What is absolute zero?
The equation to calculate the volume of a regular solid and units
What is L x W x H and cm3
A 40.5 g block of aluminum has a density of 2.70 g/mL. Find the block's volume.
What is 15 mL ?
True or False: the boiling and melting points of water will stay the same no matter how much water there is.
What is true?
Food Calories are __________ heat calories
what is 1000?
The state of matter with the lowest kinetic energy
What is a solid?
Method to calculate volume of an irregular shape, like a rock.
What is measurement after - measurement before or water displacement?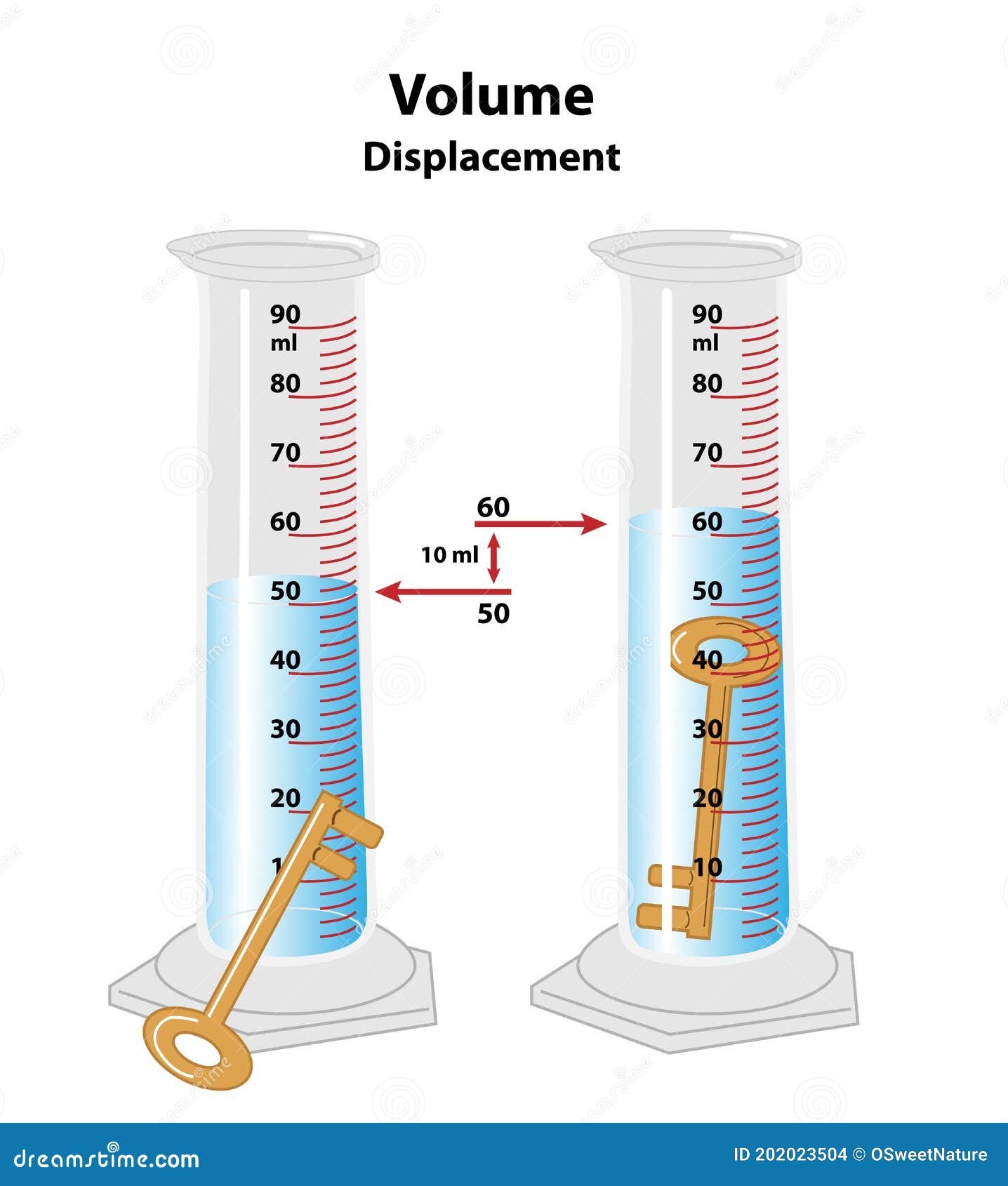
A block of wood is 3 cm on each side and has a mass of 27 g. Calculate the volume and density of the block and include units. Hint: L x W x H to find volume and d = m ÷ v
What is 27 cm3 and 1 g/cm3
As water is cooled, while ice is forming, what happens to the temperature of a solution?
What is stays the same?
OR What is remains constant?
The number of Joules in 1 heat calorie
What is 4.184?
State of matter that is electrically charged gas
What is plasma?
Density of pure water
What is 1 g/mL?
A graduated cylinder has 45 mL of water in it. A rock with a mass of 12 g is placed into the cylinder and the water level went up to 61 mL. Find the density.
What is 0.75 g/mL
Malleability is a physical property that refers to what?
What is matter's ability to be flattened without breaking?
When a candle is lit, the wick burns, the wax melts, the candle changes shape, and the air around the candle heats up. Is the reaction endothermic or exothermic?
What is exothermic