Anything that has mass and volume...
Matter
Which 2 of the following are examples chemical properties...
Flammability, Boiling, Ability to Rust, Flexibility
Flammability and Ability to Rust
What measurement of matter is the amount of space an object takes up?
Volume
A change that alters the form or appearance of a material, but does not turn the material into another substance
Physicl Change
Thermal Energy flows from ________ matter to __________ matter
Warmer to colder
The most basic building block of all matter...
Atom
Which 2 of the following are examples physical properties...
Flammability, Boiling, Ability to Rust, Flexibility
Boiling and Flexibility
Difference between mass and weight
Mass is the amount of matter in an object and is not affected by gravity. Weight is a measure of the gravitational pull on an object.
A new substance being formed is an indication of a _________ change.
Chemical
What the difference between endothermic and exothermic changes?
Endothermic change – energy is absorbed
Exothermic change – energy is released
Substances that are made of only one type of atom and are found in the periodic table..
Elements
Heterogeneous Mixture
Type of mixture that you can see the different parts
How do you find the volume of a regular shaped object?
Multiply length X width X hight
Changing State or shape are examples of ___________ changes.
Physical
What is the volume of the rock sample?
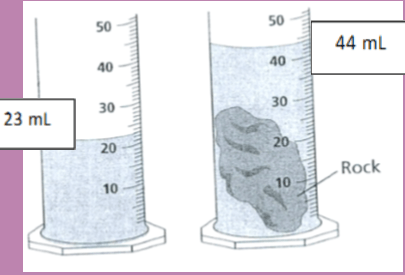
21 mL
Molecule
Atoms- same OR different- that are chemically bonded together..
Homogenous Mixture
Type of mixture where the whole thing looks the same
Explain how to find the volume of an irregular object...
DISPLACEMENT - RECORD THE STARTING WATER LEVEL. SUBMERGE THE OBJECT INTO THE WATER AND RECORD THE NEW WATER LEVEL. SUBTRACT AND YOU WILL BE LEFT WITH JUST THE VOLUME OF THE IRREGULAR OBJECT.
Buring, cooking, and photosynthesis are examples of ____________ changes.
Chemical
Find the mass of the sand that is inside the beaker in the picture below.
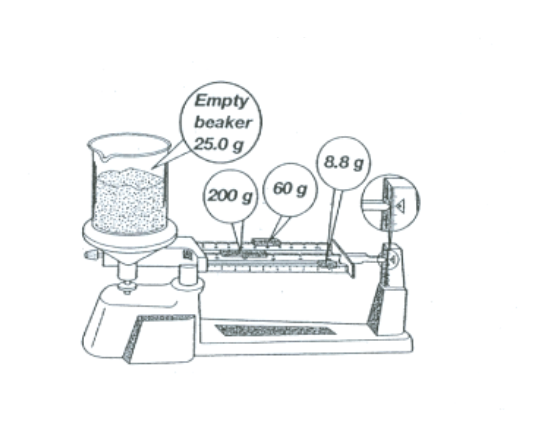
Mass = 243.8g
Compund
Two or more different types of atoms bonded together...
The steps of the Scientific Method
Observation
Question
Hypothesis
Experiemnt
Results
Conclusion
Density = mass / volume
Conservation of Mass
Matter is not created or destroyed in any physical or chemical change.
Mass of object is 240 g and the volume is 36 mL. What is the density?
240/36 = 6.67 G/CM3