Name KCl
What is Potassium Chloride?
What is the octet rule?
The octet rule states that all elements (except H and He) want to have 8 electrons in their outer shell.
Where are valence electrons found?
What is outermost energy level?
What is a "MOLECULE"?
A molecule is another name for a COVALENTLY bonded compound.
In a flame test, what color does sugar emit?
What is colorless?
What are ions?
What are charged atoms?
This is a solution that conducts electricity because of the movement of ions.
What is an ELECTROLYTE?
True or False: Phosphorus can form 5 covalent bonds because it has 5 valence electrons.
What is....False?
Materials that do not conduct electricity.
What are insulators?
This is the term given to an covalent compound formula.
What is Structural Formula?
What is the oxidation number/ionic charge for Boron?
+3
What is nonmetal?
Show how an ionic bond is formed between sodium and sulfur. Write its formula.
Na2S
This is the term used to describe the amount of energy required to break a covalent bond.
What is bond energy?
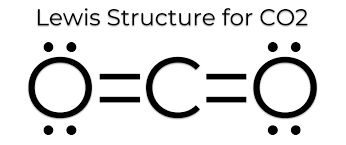
Draw the electron dot diagram for water.