In an ionic bonds, the electrons are ________
Given from the metal to the nonmetal.
How many valence electrons does nitrogen have?
5
In a Lewis Dot Strucure, each dot represents a(an)
What is valence electron
If there are two long dashes between two atoms in a structural formula, molecules of the compound contain a(an) ____________________ bond.
What is double bond
What does the state of matter symbol "s" stand for?
Solid
Typically, atoms gain or lose electrons to achieve
What is stable electronic configuaration
Metals that form ions are called _______ ions.
cations (Positive)
What two types of atoms make a covalent bond?
Nonmetals and nonmetals
The chemical formula for calcium chloride, CaCl2, shows that the compound contains two ____________________ ions for every ____________________ ion.
What is chloride ions for every one calcium ion
What does the state of matter symbol "l" stand for?
Liquid
In an Lewis Dot Structure, the symbol for an element is used to represent
What is the nucleus and all non-valence electrons
How many electrons are shared in a double bond?
4
Draw the following compound: NaOH

In a covalent bond, elements will _________ electrons.
Share
What does the state of matter symbol "g" stand for?
Gas
Elements bond to form an ________
Octet (8 electrons)
Draw the following compound: MgCl2
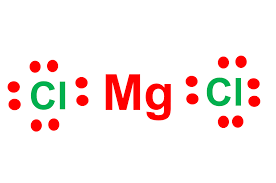
Which of the following are not a covalent bond: CO2, CH4, SO3, AlPO4, N2O3
AlPO4
In the binary ionic compound potassium bromide, KBr, the element that forms cations is ____________________.
What is K potassium
What does the state of matter symbol "aq" stand for?
Aqueous
JACKPOT!!!! IF YOU ANSWER CORRECTLY YOU GET DOUBLE THE POINTS!!! Which of the following compounds does NOT belong? H20, CO2, H2, NaCl
What is NaCl
Draw the following molecule: CO2
If there are three short dashes between two atoms in a structural formula, molecules of the compound contain a(an) ____________________ bond.
What is a triple bond
There are two additional states of matter, name one.
Plasma or Boseman-Einstein Condensates