Why do atoms give or take electrons? Why do they do that?
To have a full outer energy level.
Ionic bonds form between ______ & ______.
Metals and Nonmetals
Covalent Bonds form between ______ & ______.
Nonmetals & Nonmetals
What is the difference between ionic and covalent compounds as far as electrons are concerned?
What is the oxidation number for Calcium.
2+
Do metals tend to give away or take valence electrons?
Give away
What would be the Ionic Formula for Sodium and Sulfur?
Na2+ S2-
Draw the lewis dot structure of a covalent compounds that is composed of Oxygen & Flourine
F --- O --- F
When elements form bonds, it changes their __ properties.
physical and chemical
What is the oxidation number for Indium?
3+
Why does an ion of Magnesium (#12) have a 2+ charge?
Magnesium ions have two more protons than they do electrons.
What is the Ionic formula between Potassium and Nitrogen?
K3+N3-
How many bonds will there be between two Oxygen atoms?
2 bonds (see below)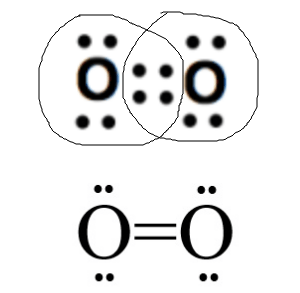
What does it mean when a molecule is polar?
One end of the molecule is + and the other side is -.
What is the oxidation number for Radon?
What charge would an ion of Nitrogen (#7) have?
-3
What holds the atoms in ionic bonds together?
The ion's opposite charges.
Ex. Na+ and Cl-...the opposite charges make the ions "stick" together.
Which of the following may be classified as a binary compound?
NH4Cl
Mg(OH)2
H2SO4
NaCl
NaCl
What is the difference between carbon monoxide and carbon dioxide?
Carbon monoxide has only one oxygen atom. Carbon dioxide has two oxygen atoms.
What would be the oxidation number for Arsenic?
3-
Will Silicon (#14) form an ion? Why/Why not?
Yes. Silicon has 4 valence electrons; it can give or take 4 electrons.
True or False?
Chlorine (#17) can form an ionic bond with all group 1 elements.
False - Hydrogen is a nonmetal and will form a covalent bond with Chlorine
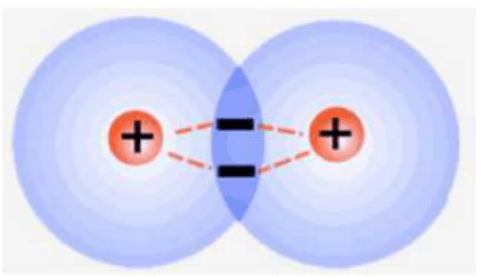
What holds the atoms in covalent bonds together?
The shared electrons are attached to BOTH nuclei
Ionic compounds are usually formed by ionic bonding between metals and nonmetals. Which of the following is not an ionic compound?
KI
MgCl2
NaBr
HCl
HCl
1+