Why do atoms give or take electrons? Why do they do that?
To have a full outer energy level.
Ionic bonds form between ______ & ______.
Metals and Nonmetals
Covalent Bonds form between ______ & ______.
Nonmetals & Nonmetals
What is the difference between ionic and covalent compounds as far as electrons are concerned?
Why don't the noble gases bond with other elements?
They have full valence shells.
Do metals tend to give away or take valence electrons?
Give away
Draw the lewis dot structure for Sodium Sulfide.
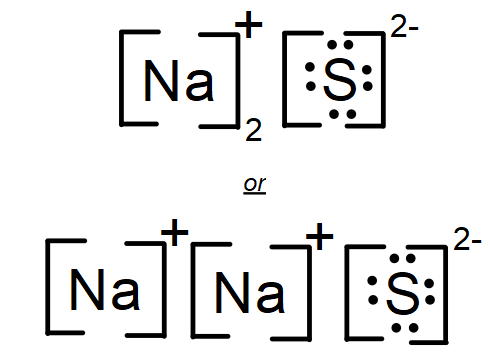
Draw the lewis dot structure of a covalent compounds that is composed of Oxygen & Flourine
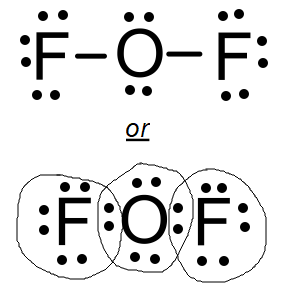
Do ionic compounds have a low or high melting point?
High melting point.
(It takes a lot of energy to break ionic bonds)
How many valence electrons does sulfur have?
6 valence electrons
Why does an ion of Magnesium (#12) have a 2+ charge?
Magnesium ions have two more protons than they do electrons.
How many ions of Potassium (#19) will there be in an ionic bond between Potassium and Nitrogen (#7)?
3
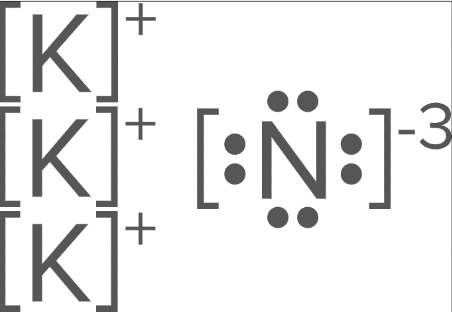
How many bonds (sharing of 2 e-) will there be between two Oxygen atoms?
2 bonds (see below)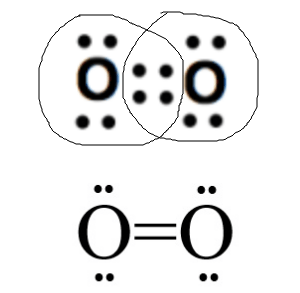
When dissolved in water, are covalent compounds good or poor conductors?
Poor
What two elements don't need 8 valence electrons?
What charge would an ion of Nitrogen (#7) have?
-3
What holds the atoms in ionic bonds together?
The ion's opposite charges.
Ex. Na+ and F-...the opposite charges make the ions "stick" together.
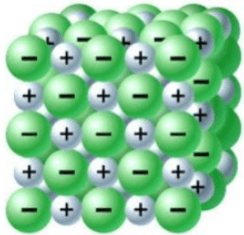
In a (an) _______________, electrons are shared.
Covalent bond
Why is the melting point of covalent compounds so low?
Why is carbon considered the backbone element?
Because it can make up to four bonds, which is the most an element can make.
An atom that has lost or gained electrons becomes a (an) _______.
ion
True or False?
Chlorine (#17) can form an ionic bond with all group 1 elements.
False - Hydrogen is a nonmetal and will form a covalent bond with Chlorine
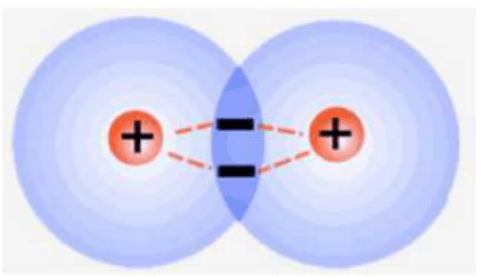
What holds the atoms in covalent bonds together?
The shared electrons are attached to BOTH nuclei
Why do ionic compounds conduct when dissolved in water?
Water separates the ionic compound into it's two ions. The ions are charged particles, and will conduct electricity in water.
OH- means what?
That it's a negative ion, so O has 7 valence electrons instead of 6