Why do atoms give or take electrons? Why do they do that?
To have a full outer energy level.
Ionic bonds form between ______ & ______.
Metals and Nonmetals
Covalent Bonds form between ______ & ______.
Nonmetals & Nonmetals
What is the difference between ionic and covalent compounds as far as electrons are concerned?
Name the ionic compound: Li2O
Lithium Oxide
Do metals tend to give away or take valence electrons?
Give away
Draw the lewis dot structure for Sodium Sulfide.
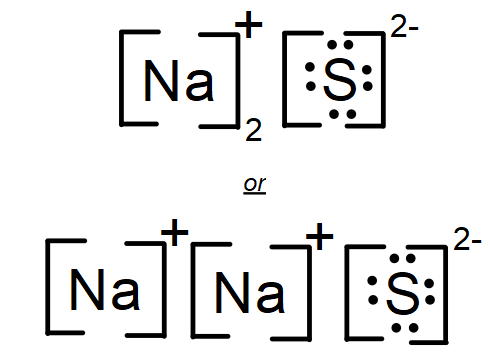
Draw the lewis dot structure of a covalent compounds that is composed of Oxygen & Flourine
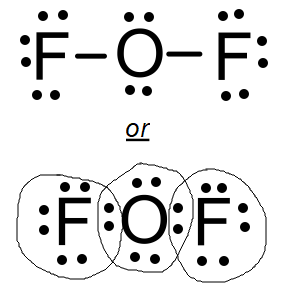
Do ionic compounds have a low or high melting point?
High melting point.
(It takes a lot of energy to break ionic bonds)
Name the covalent compound: CH4
Carbon tetrahydride
Why does an ion of Magnesium (#12) have a 2+ charge?
Magnesium ions have two more protons than they do electrons.
How many ions of Potassium (#19) will there be in an ionic bond between Potassium and Nitrogen (#7)?
3
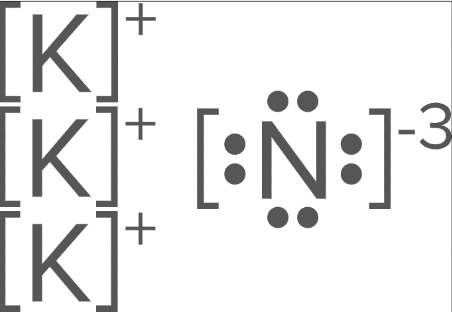
How many bonds (sharing of 2 e-) will there be between two Oxygen atoms?
2 bonds (see below)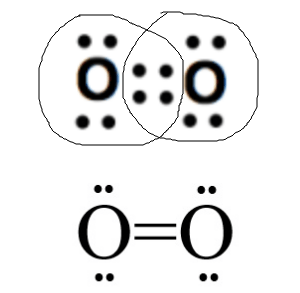
When dissolved in water, are covalent compounds good or poor conductors?
Poor
Name this compound: H2S
dihydrogen monosulfide
What charge would an ion of Nitrogen (#7) have?
-3
What holds the atoms in ionic bonds together?
The ion's opposite charges.
Ex. Na+ and F-...the opposite charges make the ions "stick" together.
Name 3 diatomic molecules.
(Write the chemical formula. )
Potential Aanswers:
H2, N2, F2, O2, I2, Cl2, Br2
Why is the melting point of covalent compounds so low?
Name this compound: CuCl2
Copper Chloride
Will Silicon (#14) form an ion? Why/Why not?
Silicon has 4 valence electrons; it takes a lot of energy to give or take electrons, so it will share electrons instead. No ion will form.
True or False?
Chlorine (#17) can form an ionic bond with all group 1 elements.
False - Hydrogen is a nonmetal and will form a covalent bond with Chlorine
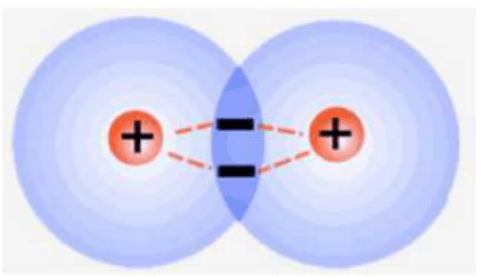
What holds the atoms in covalent bonds together?
The shared electrons are attached to BOTH nuclei
Why do ionic compounds conduct when dissolved in water?
Water separates the ionic compound into it's two ions. The ions are charged particles, and will conduct electricity in water.
Name this compound: PbNO3
Lead Nitrate