What particles within an atom are involved in bonding?
What are valence electrons?
When Oxygen becomes an ion, it has this charge.
What is 2-?
These types of elements are involved in covalent bonding.
What are nonmetals?
This type of bond is represented below. 
What is a single covalent bond / covalent bond?
An ionic bond is usually between what two types of elements.
What is a metal and a nonmetal?
When Aluminum develops a charge, it has this charge:
What is 3+ ?
Atoms that are covalently bonded do this with their electrons.
What is share?
In the diagram for H2O, there are this many shared/bonded electrons around the central atom.
What is 4 electrons / two pairs of electrons?
This type off element is generally referred to as an 'electron donor'.
What is a metal?
This is the formula for the salt made from Calcium (Ca) and Chlorine (Cl):
What is CaCl2 ?
When modeling covalent molecules with Lewis Dot Diagrams, this is represented by drawing a line between two atoms.
What are bonded electrons / a bonded pair?
There are this many paired electrons around the molecule, and this many bonded pairs.
What is one paired set, and three bonded sets?
In an ionic bond, this is what happens to valence electrons:
What is transferred/lost and accepted/gained?
This is the formula for the salt made from Barium (Ba) and Selenium (Se):
What is BaSe ?
This element is the most electronegative and hogs electrons in a covalent bond.
What is Fluorine?
Looking at the diagram for CO2, this many electrons are being shared between carbon and the other atoms.
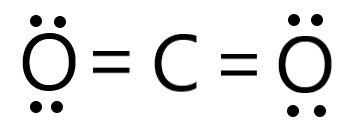
What is 8 electrons?
This type of ion tends to gain electrons, and the end of it's name is replaced with -ide.
What is an anion?
This is the formula for the salt made from Magnesium (Mg) and Phosphorous (P):
What is Mg3P2 ?
These 'multi'-types of covalent bonds are the strongest kind and the hardest to break.
What are double and triple covalent bonds?
In the molecule H2CNH, there are this many single bonds present.
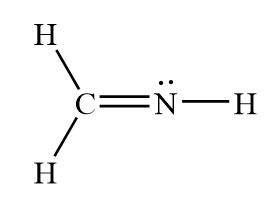
What is 3 single bonds?
FINAL JEOPARDY: This easily breakable type of element is semi-conductive to electricity.
What is a metalloid?