How many valence electrons are in Al?
What is 3?
What type of elements form covalent bonds?
What are nonmetals?
How many electron regions does CS2 have?
What is 2?
What type of elements forms ionic bonds?
What is a metal and nonmetal?
What subatomic particle
Draw the LDS for H
What is
How many bonds can P make?
What is 3?
What is the molecular geometry of CF4?
What is tetrahedral?
What is the chemical formula of Li + F?
What is LiF?
Covalent Bonds _________ electrons where as ionic bonds _________ electrons.
What are share and transfer?
General rule that states that atoms want 8 valence electrons to become like noble gases.
What is the octet rule?
What is 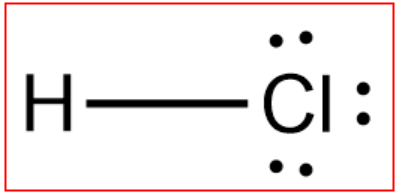
What is the molecular geometry of one of the carbons?
What is trigonal planar?
Metals form ______ ions and nonmetals form_______ ions.
What is positive (cation) and negative (anion)?
What group of atoms don't tend to form ionic bonds?
What is the Carbon group?
Draw the dot structure of O.
What is 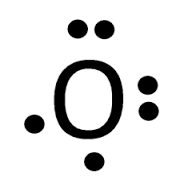
Draw the LDS for H2S
What is
What is the molecular geometry of SF2
What is bent?
What is the chemical formula of Mg + P?
How many electrons will Sr lose to become an ion?
What is 2
What is wrong with LDS (B)
Hydrogen has too many bonds. 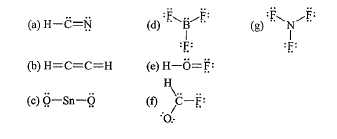
Draw the LDS for C2O2H3
What is...
What is the molecular geometry of NH3 and how many lone pairs does it have?
What is trigonal pyramidal and 1 LP?
What are the charges of each ion in Na2SO4?
What is Na1+ and SO42-?
What is the exception to the octet rule found in SF5?
What is an expanded otect?