What happens to electrons in an ionic bond
What is a transfer?
A covalent bond is between 2 or more ________ where electrons are _______.
A. nonmetals; shared B. nonmetals; transferred
C. metals; shared D. metal and nonmetal; transferred
A. nonmetals; shared
What is electronegativity a measure of?
An atoms attraction to electrons
This number of bonding sites that hydrogen has.
What is 1?
Double to, or lose 200 pts: What number of electrons will H2 have?
What's the IUPAC (official) name for CaCl2?
A. Calcium dichloride B. Calcium chloride
C. Dichloride calcium D. Chloride calcium
B. Calcium chloride
Write the IUPAC (official) name for N3O7.
Trinitrogen Heptoxide
What's the difference between how electrons are shared in a polar vs a nonpolar covalent bond?
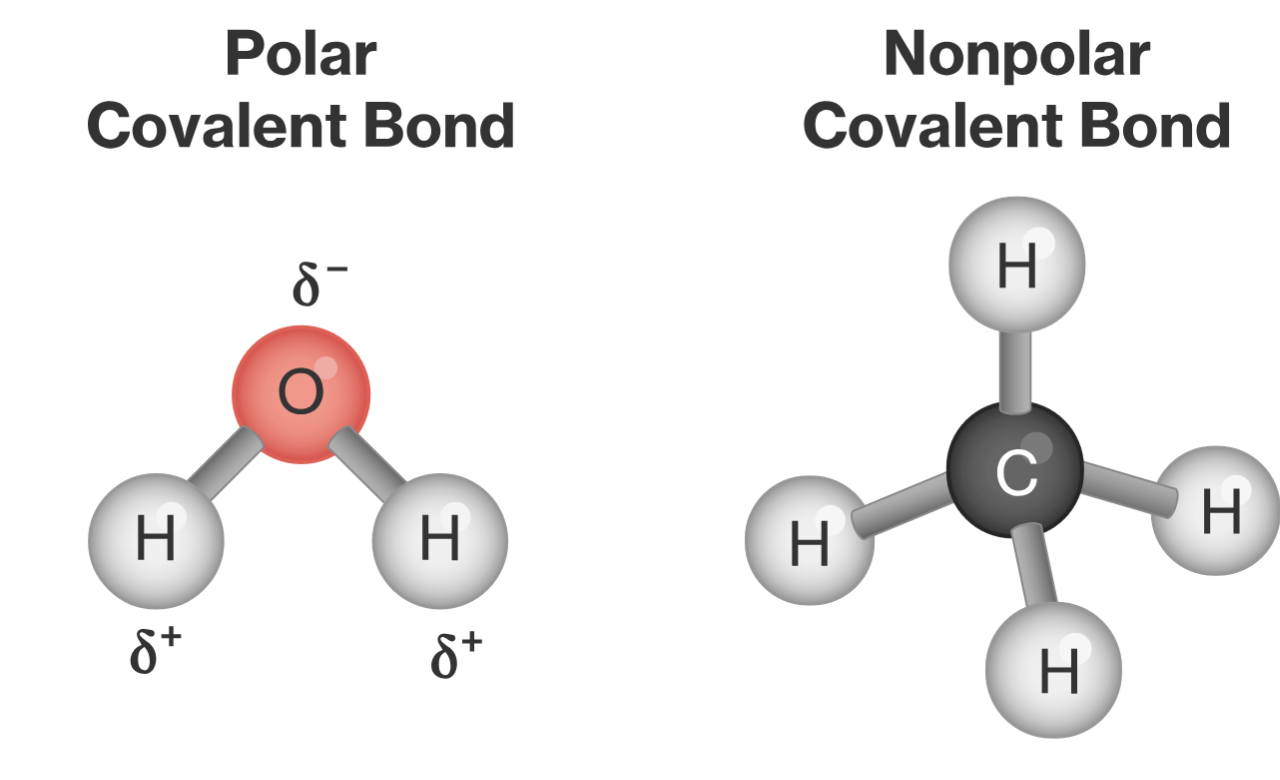
Polar covalent bonds have atoms with different electronegativities, so one atom pulls the electrons closer.
In a covalent bond, atoms have similar electronegativities and share their electrons evenly.
Make O2
Hint: Remember that oxygen makes two bonds with itself!

This is the IUPAC name for K3(PO4).
Potassium phosphate
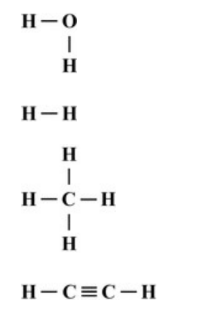
What is the correct Lewis structure for H2?
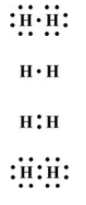

Double your points: How many electrons does each atom donate?
Polar molecules are normally shaped ________ and nonpolar bonds are __________.
A. symmetrically (even); non-symmetrically (uneven)
B. non-symmetrically; symmetrically
B. non-symmetrically; symmetrically
Make N2.
Hint: Remember that nitrogen makes three bonds with itself!
Which compound is not ionic?
A. FeCl3 B. N2O3 C. MgO D. NaF
B. N2O3
Which of the following are molecular compounds? (Choose all that apply)
A. Fe2S3
B. CaCl2
C. H2O
D. NO2
E. CO2
F. KI
C. H2O
D. NO2
E. CO2
Which of the following represents a polar molecule? Hint: Polar molecules are nonsymmetrical
Make methane (CH4) and name the geometric shape and bond angle
Tetrahedral - 109.50
Which chemical in the table above has an incorrect IUPAC name?
Hint: only use prefixes when naming binary covalent bonds.
3 CaCl2
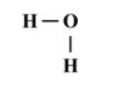
What property of water allows it to be the universal solvent?
What is polarity?
Which of the following represents a polar covalent molecule? Remember that polar means "different"
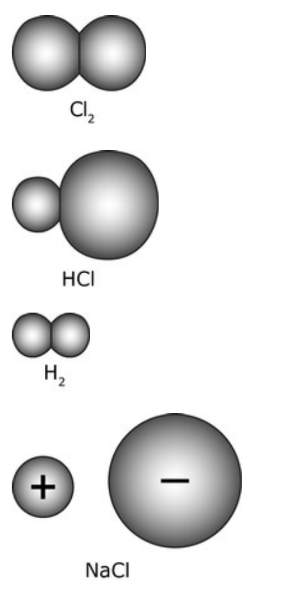
B. HCl
Make sulfur hexachloride
1. Write the chemical formula
2. Describe geometric shape
3. Give bond angle

1. SCl6
2. Octahedral
3. 900