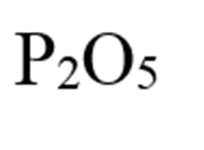
What is diphosporous pentoxide?
The Lewis dot or electron dot diagram for aluminum has how many dots around the element symbol?
What is 3 dots to show the number of valence electrons? 
Atoms that have this type of charge gain electrons.
What is negative?
The two elements that are exceptions to the octet rule
WHAT ARE HYDROGEN AND HELIUM?
NaBr
What is sodium bromide?
Chemical formula created when aluminum and oxygen bond.
What is  ? Make certain you can name this and identify if it is ionic or covalent!
? Make certain you can name this and identify if it is ionic or covalent!
Positive ions are called this.
CaBr2
What is calcium bromide?
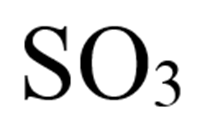
What is sulfur trioxide?
 ?
?
Magnesium ions will have this type of charge.
What is +2?
This term is used to define how well an atom can attract an electron to it (related to the amount of energy required to remove an electron from the outer shell).
What is electronegativity?
Molecules are this type of compound.
What is covalent?
When atoms try to get a full outer shell of electrons (usually 8 electrons).
What is the octet rule?
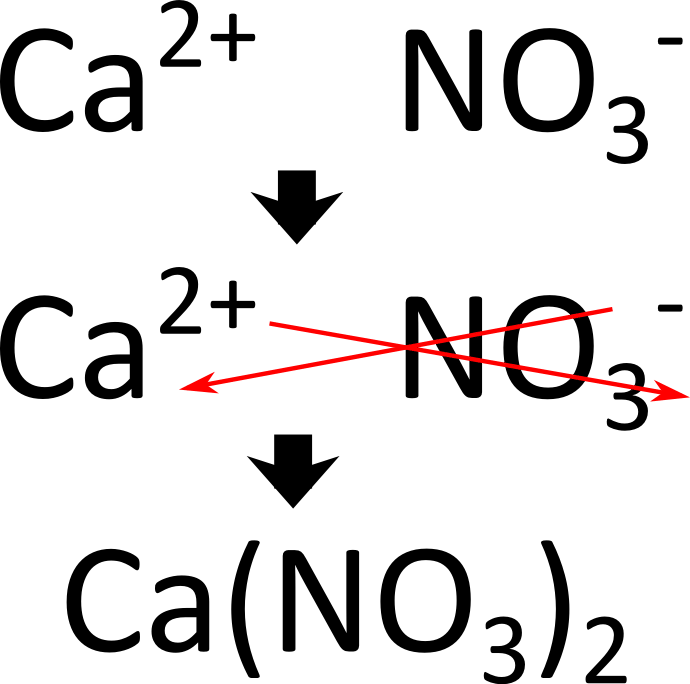 ?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?
?
In case you forgot how we get the final formula the image includes a step by step of the criss cross method!!! Review your notes/ask questions prior to the test if you need to.
Bonus question:
How many elements are present?
How many atoms of each element are present?Non metals tend to form this type of ion.
What is negative/anions?
We call covalently bound groups of nonmetals which carry a charge, _____________.
What are polyatomic ions?
NAME THE 7 DIATOMIC (SUPER 7) MOLECULES
HYDROGEN, NITROGEN, OXYGEN, FLUORINE, CHLORINE, BROMINE AND IODIDE
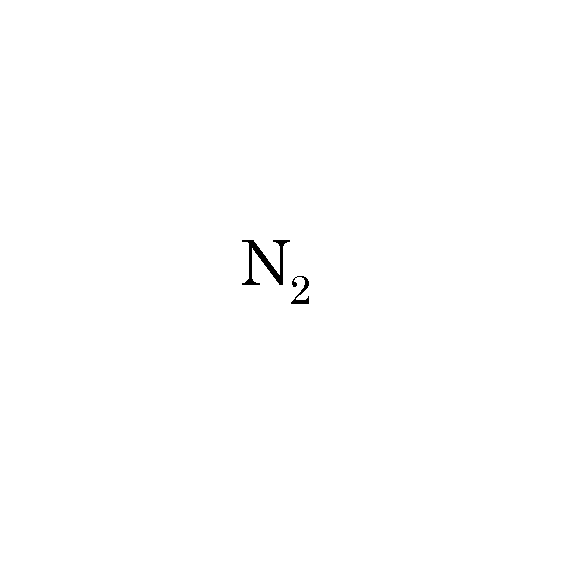
What is nitrogen?
 ) is an example of a(n) _________ compound.
) is an example of a(n) _________ compound. NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?
NOTICE: ammonium is a positive polyatomic ion and nitrate is a negative polyatomic ion. This is a tricky one but you do need to use the resources you are given!
Bonus question:
How many nitrogen are in this chemical formula?Elements in group 15 tend to have this type of charge.
What is negative 3 charge?
A nonmetal or nonmetal group bound to a metal makes up this type of compound.
What is an ionic compound?
The Roman numeral in a compound tells you this.
What is the charge (oxidation number) of the metal?