This determines reactivity
What are valence electrons
This increases as you descend a group
What is energy levels
protons also accepted
Charge of each subatomic particle
What is:
Protons are positive
Neutrons are Neutral
Electrons are negative
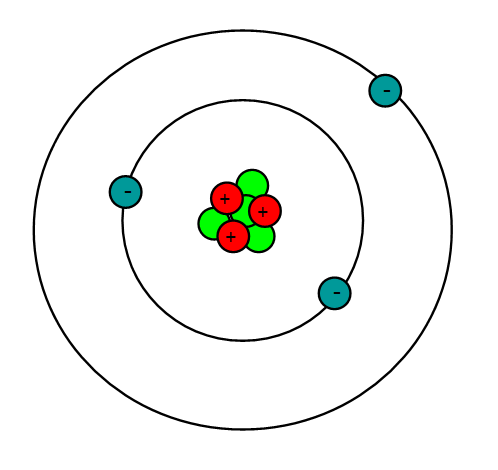
What is Lithium
Location of most reactive elements
Where is 1A and 7A
This tells you number of energy levels
What is period
Electrons live here
What is Electron Cloud
Similar reactivity to Aluminium but less atomic mass
What is Boron
If elements have the same number of this, they will have the same reactivity
What are valence electrons
You have 7 of these on the periodic table, and on your class schedule
What is periods
Mass of an atom with 15 protons, 15 electrons, and 17 neutrons
What is 32 amu
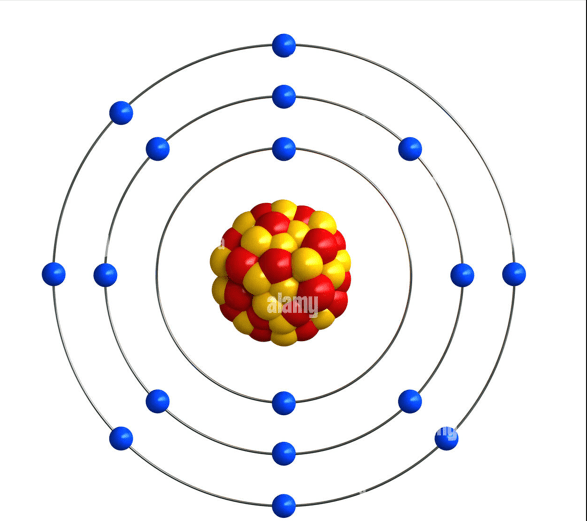
What is Chlorine
Name two elements with similar chemical properties to Arsenic
Nitrogen
Phosphorus
Antimony
Bismuth
Tells you number of valence electrons
What is group (A) number
This is equal to protons
What is number of electrons
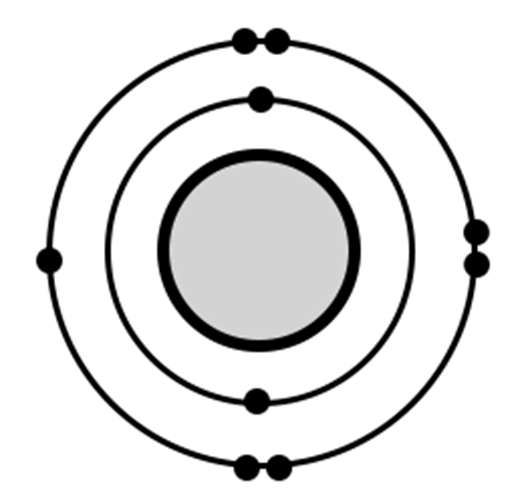
What is Fluorine
Name of least reactive group and why
What is Carbon group or 4A
Carbon has to either gain 4 electrons or lose 4 electrons to react.
The location of Germanium (think of a grid)
What is group 4a and period 4
Remains constant for an element; identity
What is protons
What is Barium
The element Uranium has this many energy levels
What is 7
