What are the units of mass?
What is g, mg, kg
Provide the measurement to one uncertain digit (Remember to include units):
What is 1.65 cm
Solve for the mass of an object that has a density of 0.92 g/mL and volume of 1.87 mL:
What is 1.72 g
Convert 0.0391 to scientific notation
What is 3.91 x 10-2
Convert 180 mm to m:
What is 0.180 m
What are the units of volume?
What are mL, cm3, m3
What is the measurement to one uncertain digit (remember to include units in mL)::max_bytes(150000):strip_icc():format(webp)/meniscus01-58b5b2c03df78cdcd8ab8299.png)
What is 24.0 mL
What is the density of a 13 g cube that has a side length of 2.5 cm?
What is 0.832g/cm3
Convert 3.1 x 10-4 to standard notation
What is 0.00031
Convert 7.8 in to cm:
What is 19.8 cm
What are the units of Density?
what are g/mL, g/cm3
What is the following measurement read to one uncertain digit (make sure to include units in mL in your answer):
:max_bytes(150000):strip_icc():format(webp)/meniscus04-58b5b2e65f9b586046bb2723.png)
What is 2.65 mL
An empty beaker is put on the balance and measures 85.0 g. It is filled with 50 mL of liquid and now measures to be 128.0 g. What is the density?
What is 0.86 g/mL
Convert 0.000080 to scientific notation
What is 8.0x10-5
Convert 3.2 hours to seconds:
What is 11,520 seconds
What is the equation for Density?
What is D= m/v
What is the volume of the toy figure in mL? Make sure to include one uncertain digit: 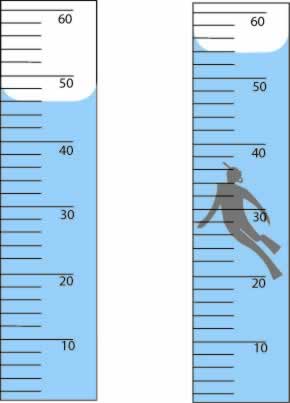
What is 8.0 mL
Solve: 7.1 x 105-4.9x105. Put your answer in scientific notation
What is 2.2x105
Convert 7.300128 x 105 to standard notation:
What is 730012.8
Convert 134.2 cm to mi:
What is 0.0008339 mi
How would you set up the equation to solve for a missing volume when you are given density and mass?
What is V= m/D
You are taking the mass of an object using a triple-beam balance and see the three measurements on each beam. What is the mass of the object in g to one uncertain digit?
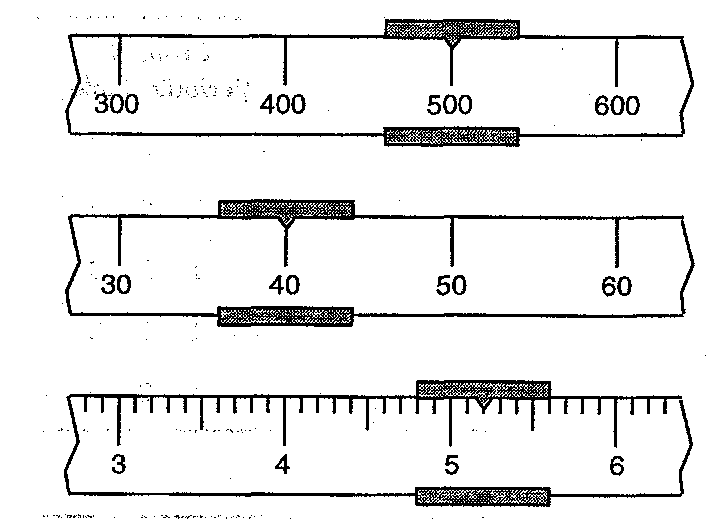
What is 545.20 g
Solve: (2.2x106) x (1.3x10-3). Put your answer in scientific notation.
What is 2.86x103
Convert 3,200,000 to scientific notation
What is 3.2x106
Determine how many kg are in 157.2 mL water (Note: the Density of water is 1 g/mL)
What is 0.1572 kg