How many significant figures is in the number: 0.006070
4 sig figs
Based on this diagram, which isotope of copper is least abundant?
Copper-65
How many valence electrons does chlorine have?
7 VE's
Write in scientific notation with 4 significant figures:
12,785,000
1.279 x 107
How many neutrons is in: 13C
7 neutrons
What is the electron configuration of argon.
1s22s22p63s23p6
Convert 12.60 mL --> L
0.0126 L
Calculate the average atomic mass of Boron. (4 sig figs)

10.83 amu
What is the noble gas configuration of arsenic?
[Ar]4s23d104p3
What is the mass of an atom with 8 electrons, 8 protons, and 10 neutrons.
18 amu
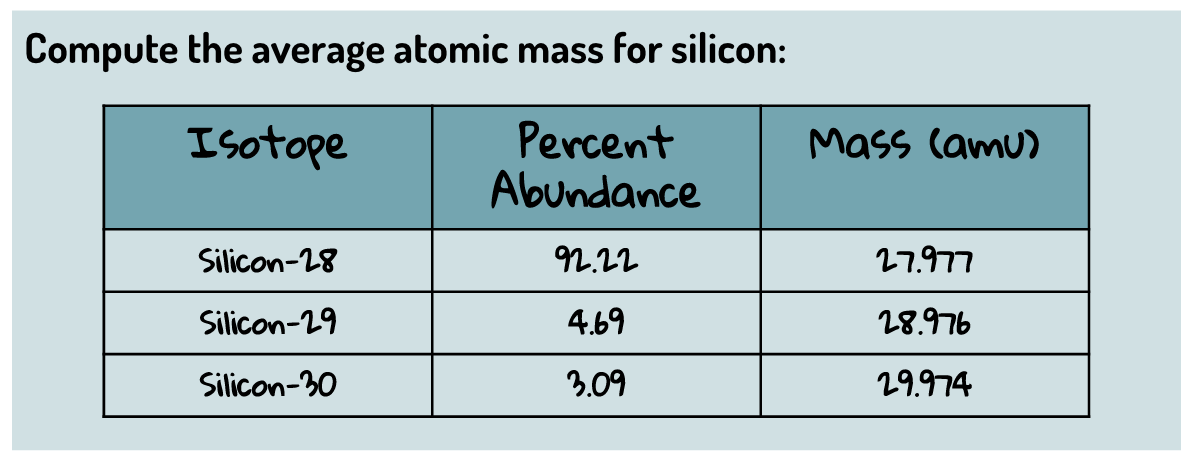
(4 sig figs)
28.08 amu
Draw the electron orbital diagram for magnesium.

Calculate the mass percent of hydrogen in hydroxide (OH) if hydrogen has a mass of 2.0 g and the total mass of OH is 17.0 g. (3 sig figs)
11.8% of H in OH
Nitrogen has two isotopes, N-14 and N-15. If the average atomic mass is 14.007 amu, what is the abundance of isotope N-15? (Write answer with 2 sig figs)
0.70%
Atoms are more stable when their outer energy level is full. For most elements, this means having how many electrons in their outer energy level?
8 electrons (octet)
Stability here just means the electrons are in a lower-energy, more comfortable arrangement.