Name the 3 labels on this diagram of an atom.

a = electron
b = neutron
c = protron
Isotopes of a given element will have a different number of what sub-atomic particle:
a. electrons
b. protons
c. neutrons
A different number of neutrons
What causes radioactive decay?
A radioisotope with an unstable nucleus.
Name the 2 types of radiation.
Non-ionising radiation and ionising radiation
Name the main kid in Toy Story.
Andy
How many protons in carbon?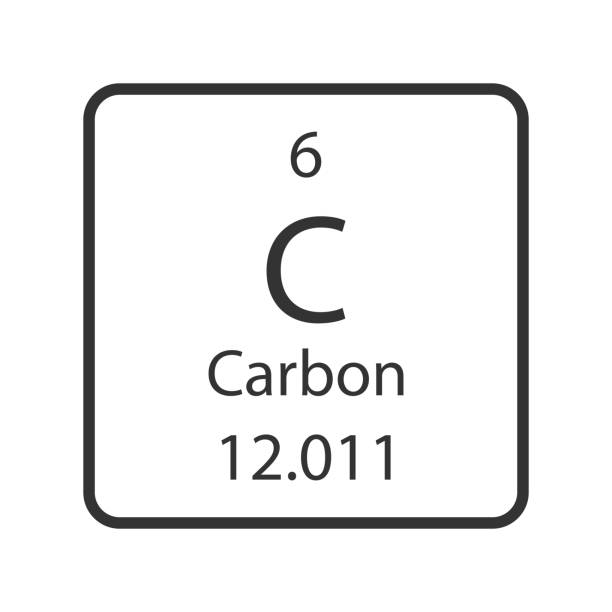
6
If Krypton-80 has 36 protrons how many neutrons does it have? 
44 neutrons
Rank the types of radioactive decay from strongest to weakest penertrating power. (e.g. going through paper vs tinfoil)
Gamma, beta, alpha
What is ionisation?
Radiation that causes atoms to become ionised/ ions (removes an electron from the atom to make it positive).
Who won the 2024 NRL championship?
Penrith Panthers
What is the atomic mass for helium?
4.003
What is Plutonium-260 atomic mass?
260 amu
Identify the type of decay in the equation below
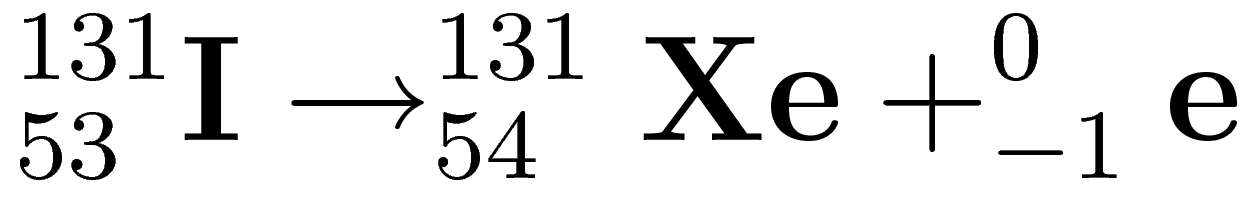
Beta decay
What type of radioactive decay is this?
.png)
Alpha decay
Finish the lyric from 'Bye Bye Bye' by NSYNC:
Don't wanna be a fool for you
Just another player in your game for two
You may hate me, but ....
It ain't no lie
Baby, bye, bye, bye (bye, bye)
Calculate the number of neutrons in oxygen-16.
Neutrons = mass - protons = 16 - 8 = 8 neutrons
Knowing that carbon has 6 protons, name this isotope:

Carbon-14 or C-14
Explain the changes that occur in the parent nucleus during alpha decay.
Loss of 2 protons and 2 neutrons (an alpha particle)
How are an alpha particle and a helium atom different?
What is a helium atom has electrons, but an alpha particle does not (hence the +2 charge)
What is the name of Beyoncé and Jay-Z's first-born?
Blue Ivy
Calculate the number of neutrons in hydrogen-1.

Neutrons = mass - protons = 1 - 1 = 0 neutrons
Identify the number of protons, neutrons and name of this isotope.

92 protons, 146 neutrons, Uranium-238
Rubidium-87 (Rb has 37 protons) undergoes beta decay (neutron turns into a proton and releases an electron). Strontium (Sr) has 38 protons.
Write an equation representing this process.
Example:
What is the half-life of the isotope in the graph below?

6 days
Name four of the seven horcruxes in Harry Potter.
Double points if you get all 7 horcruxes!
Tom Riddle's diary, Marvolo Gaunt's ring, Slytherin's locket, Hufflepuff's cup, Ravenclaw's diadem, Nagini (the snake), and Harry Potter (an accidental Horcrux).