What is deionization
When atmospheric pressure = vapor pressure
What is boiling point
This is the phase change from solid that is ENDOthermic
What is melting
This is the definition of a triple point
What is the point at which solid, liquid and gas exist in equilibrium
This is a collision that loses no energy
What is an elastic collision
This is the attraction between liquid and the surface of a solid
What is capillary action
These are the two phase changes that skip liquid
What are sublimation and deposition
These are the definitions of exo- and endothermic changes
exo- releases energy
endo - requires energy
Name 4 of the 7 diatomic molecules
What are H2, N2, O2, F2, Cl2, Br2, I2
This phase change from liquid is exothermic
what is freezing
Glass is defined as this type of solid
What is amorphous
This is the definition of a supercritical fluid
What is when liquid and gas are indistinguishable
Assume all gases are at the same temperature this gas is moving the fastest
What is hydrogen gas (H2)
What phase has less kinetic energy than liquid
What is solid
This is the Hfus of water
What is 334 J
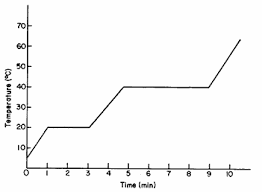
On the diagram this is the melting and boiling points
What are MP = 20C and BP = 40C
If it takes 5850J to boil 15g of hexane this is the Hvap (use the correct units)
What is 390 J/g
In a liquid these particles have the highest KE and the lowest intermolecular attraction
Name all 4 subtypes of crystalline solid
What are ionic, covalent network, metallic and covalent molecular
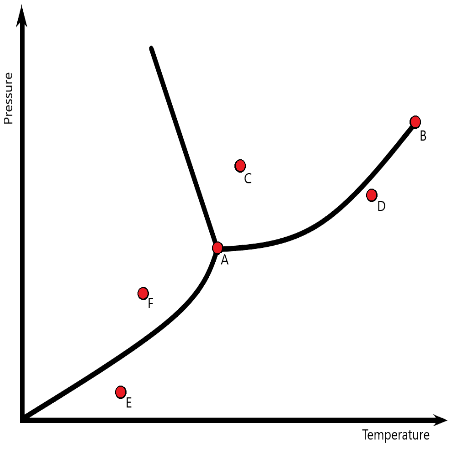 On the diagram these are the labels A - F
On the diagram these are the labels A - F
what are A = triple point B = Critical point C = liquid D = gas and E = gas F = solid