You are seeing a patient with longstanding, difficult to control hypertension in CKD clinic. While you are attempting to counsel the patient regarding his target BP, he grows frustrated and states, “every time I go to the doctor, my BP is never good enough for you guys, and you just want to throw more medication at me.”
Which of the following strategies, if used as a single intervention, is most likely to improve the patient’s engagement with his treatment plan?
A. Have the patient begin self-monitoring BP at home and ask him to send BP readings to the office in 2 weeks
B. Acknowledge the fact that many patients find taking BP medications exactly as prescribed challenging and ask him how many doses of his BP medication he estimates he is missing in a week
C. Assess patient’s medication regimen to determine whether any medications can be switched to a single pill combination agent
D. Counsel patient on nonpharmacologic strategies that can improve BP control, including following the DASH diet, regular physical activity, smoking cessation, and weight loss
What is (A)
Current guidelines now support increased use of home BP monitoring/self-monitoring for management of hypertension. Self-monitoring of BP serves the dual purpose of assessing the patient’s BP control in his home environment to evaluate for white coat effect and making him an active participant in his care, which may improve engagement. Asking about missed doses of medications may help identify issues with adherence but will not increase his engagement, if employed as a single intervention. Similarly, adjusting medications to a single pill combination drug may improve medication adherence but not necessarily engagement. Lifestyle modification is an important part of BP control, but this patient is described as having “longstanding” difficulty in controlling BP and therefore has likely received this counseling before. Home BP monitoring provides the patient with feedback regarding their efforts at BP control, something that lifestyle modification does not provide.
A 64-year-old woman with a past medical history of cholelithiasis and hypertension is admitted to the medical intensive care unit for septic shock. A contrast computed tomography of the abdomen and pelvis is suggestive of bile duct dilatation suspicious for stones in the common bile duct, which is confirmed in the magnetic resonance cholangiopancreatography. She receives fluid resuscitation with 5 L of isotonic fluids and is started on norepinephrine infusion through a central venous catheter. She has anuric acute kidney injury but has not received any renal replacement therapy. An endoscopic retrograde cholangiopancreatography is performed with extraction of common bile duct stones, and she is on broad-spectrum antibiotics. Her hemodynamic status improves over the next 2 days, and she is weaned off the norepinephrine infusion. She has 2+ pitting presacral edema on examination. Her serum creatinine level is currently 4.2 mg/dl, and her baseline serum creatinine before hospitalization was 0.8 mg/dl. Her serum sodium concentration is 151 mEq/L.
Which of the following best explains the cause of her hypernatremia?
A. Total Total body free-water deficit due to lack of access to water
B. Total body sodium and water gain with tonicity imbalance between fluid losses and fluid gains
C. Decreased metabolic water production from sepsis-induced mitochondrial dysfunction
D. Vasopressin resistance
What is (B)?
Hypernatremia in the ICU occurs because of a complex pathophysiology and is not completely explained by lack of intake of water. The presence of edema in these patients represents a total body excess of water compared with the nonedematous state. Hence, there is no total body water deficit in this patient, and answers A and C are incorrect. The presence of edema represents an overall sodium gain, which may be multifactorial in the setting of activation of renin-angiotensin-aldosterone system, acute kidney injury, and replacement of hypotonic fluid losses (sweat, feces, etc.) with isotonic replacement fluids. Hence, answer B is the correct answer. There is no evidence of vasopressin resistance in this anuric patient, so answer D is incorrect. Free-water supplementation will likely improve the hypernatremia but may potentially worsen volume overload, especially if the acute kidney injury has not recovered. It is unclear whether such free-water supplementation will improve or worsen mortality, as no such randomized controlled trial has been conducted to answer this question.
A 30-year-old man with known polycystic kidney disease (PKD) now has a GFR of 20 ml/min per 1.73 m2. He
wishes to be referred for pre-emptive transplantation and avoid dialysis. Both parents have hypertension. He has
a sister who is interested in donating a kidney.
In which ONE of the following settings should you refer this family for genetic testing by direct gene
sequence analysis of the PKD1 and PKD2 genes?
A. Neither parent has kidney cysts. The sister is aged 24 years and has no cysts seen on ultrasonography.
B. The patient’s father has PKD. The sister is aged 24 years and has no cysts seen on ultrasonography.
C. The patient’s father has PKD. The sister is aged 40 years and has no cysts seen on ultrasonography.
D. The patient’s father has PKD. The sister is aged 24 years and has a single cyst seen on magnetic resonance imaging. Genetic testing of your patient did not reveal a previously described mutation.
What is (B)
B. The patient’s father has PKD. The sister is aged 24 years and has no cysts seen on ultrasonography
In the absence of a well established family history of PKD, it is likely that the proband has a new mutation and the risks of the sister having PKD are very low (pretest probability is approximately 1 in 5000). In the absence of cysts seen on ultrasonography, her need for genetic testing is (theoretically) not necessary (answer A is incorrect). In the setting of a documented family history, although the 24-year-old sister has no cysts seen on ultrasonography, she still has a signi cant chance of having PKD (her pretest probability of carrying a PKD gene is 1 in 2). She therefore should undergo genetic testing (answer B is correct). That risk is much lower at age 40 years in the absence of cysts seen on ultrasonography and genetic testing is (theoretically) not necessary (answer C is incorrect). For answer D, genetic testing is necessary because the presence of a single cyst seen by magnetic resonance imaging does not establish a diagnosis of PKD. However, because the proband does not harbor a known gene mutation, genetic testing must be performed by linkage analysis rather than direct gene sequencing (answer D is incorrect).
A 61-year-old woman with ESRD caused by diabetic nephropathy and congestive heart failure is seen during HD. This is her first outpatient dialysis treatment since being discharged from the hospital after treatment for pneumonia. While hospitalized, declining platelet counts were observed, and she experienced thrombosis of her arteriovenous graft, prompting suspicion for heparin-induced thrombocytopenia (HIT). She underwent successful thrombectomy. Exposure to heparin was eliminated and testing for HIT was ordered, the results of which remained pending at the time of discharge.
The patient reports that she is feeling back to baseline. She is currently dialyzing with a heparin-free circuit. The nurse reports that she clotted her dialyzer earlier in the treatment, and the circuit was replaced. Her current medications include lisinopril, atorvastatin, and insulin glargine.
Her BP is 135/72 mm Hg and heart rate is 90/min. Breath sounds are diminished at the right base. Cardiovascular examination reveals regular rate and rhythm. Mild bilateral lower extremity edema is present.
Laboratory data:
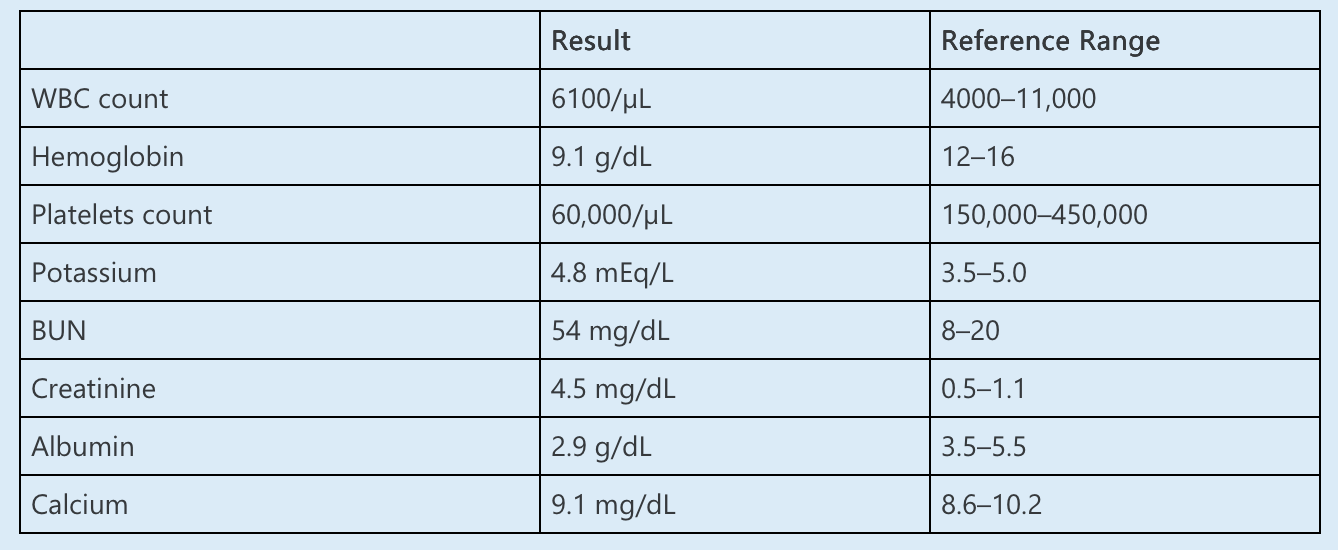
Antibodies against platelet factor-4 (PF4)–heparin complex return positive.
Which one of the following is the next BEST STEP in management of this patient?
A. Apixaban
B. Enoxaparin during dialysis
C. Citrate regional anticoagulation during dialysis
D. Isotonic saline flushes during dialysis
E. Argatroban during dialysis
What is Apixaban?
Apixaban should be initiated to prevent systemic thrombosis in this patient with ESRD, HIT, and previous thrombosis.
HIT is a relatively uncommon disorder that may occur after exposure to either unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH). There are two forms of HIT. Type 1 HIT is related to non-immune platelet aggregation that results in a mild, transient decrease in platelet count. The platelet count nadir is typically >100,000/µL, occurring 24–48 hours after exposure to heparin, followed by gradual recovery to baseline. Type 1 HIT is not associated with bleeding or thrombosis, and heparin therapy may be continued. Type 2 HIT results from antibody formation targeting PF4 complexed to heparin, leading to platelet activation and thrombosis. It is defined by decline in platelet count with absolute thrombocytopenia or a ≥50% decrease in platelet count occurring approximately 5 days after exposure to heparin, although faster onset can be seen in cases in which there has been previous exposure. Platelet count nadirs are typically in the range of 50,000–80,000/µL. Type 2 HIT, which carries up to a 30% mortality rate, occurs in 2–3% of patients exposed to UFH and <1% of patients exposed to LMWH. All heparin therapy must be discontinued, including devices that may be heparin-coated, such as IV catheters. Because patients remain at increased risk for thrombosis despite discontinuation, systemic anticoagulation with an alternative to heparin is mandatory. Up to 50% of patients may develop thrombosis within 30 days after diagnosis, so systemic anticoagulation is usually continued for at least 1 month in the absence of a thrombotic event and for ≥3 months if an event has occurred. Critically ill patients and those with limb or life-threatening thromboembolism should be treated initially with parenteral agents such as argatroban or bivalirudin. Warfarin and direct oral anticoagulants (DOACs) are options for stable patients. However, warfarin is contraindicated until the platelet count has recovered and requires overlap with a suitable agent to avoid transient thrombophilia. In this patient, who does not require hospitalization for IV therapy, apixaban is a reasonable alternative.
A 65-year-old male with a history of hyperlipidemia is evaluated for worsening hypertension and shortness of breath. He was diagnosed with hypertension 1 month ago and was started on losartan, amlodipine, and chlorthalidone. He also takes atorvastatin. Standardized office BP is 165/92 mm Hg. BMI is 29 kg/m2. Laboratory data are significant for serum potassium 5.0 mmol/L (upper limit of normal 5.2 mmol/L). Serum creatinine is 2.0 mg/dl (baseline 1.0 mg/dl). Chest x-ray reveals bilateral infiltrates. Computed topography imaging reveals right renal artery with 80% stenosis and left renal artery with 30% stenosis.
Which of the following is the next best step in management?
A. Renal artery revascularization
B. Initiate spironolactone
C. Obtain 24-hour ambulatory BP monitoring
D. Obtain aldosterone-renin ratio
What is (A) Renal artery revascularization
This patient has renal artery stenosis with a high-grade stenosis in the right renal artery. He also has resistant hypertension, evidence of pulmonary edema, and worsening kidney function. These are indications for revascularization, making choice A correct.
Initiating spironolactone would not be ideal in the setting of worsening kidney function. Also, there is no need to obtain 24 hour ambulatory BP monitoring and aldosterone-renin ratio in the setting of establish renovascular disease.
A 30-year-old pregnant woman at 32 weeks of gestation is referred by her obstetrician to the nephrology clinic because she has been complaining of excessive thirst and frequent urination for the past several weeks. She has been drinking five to six bottles of water every day and has to use the restroom to urinate every 2 hours. A basic metabolic panel sent 2 days before the visit reveals that her plasma sodium concentration is 146 mEq/L, her serum creatinine levels are 0.6 mg/dl, her serum blood urea nitrogen concentration is 14 mg/dl, and her serum glucose concentration is 146 mg/dl. Her pregnancy has been complicated by gestational diabetes mellitus, and she is currently on insulin therapy. Before the pregnancy, she had a history of drinking three to four bottles of water every day and urinating five times a day; however, she did not have a prior diagnosis of diabetes mellitus. Urine tests revealed urine osmolality of 220 mOsm/kg, urine sodium of 35 mEq/L, and 24-hour total collection of 3.5 L of urine.
What is the most likely cause of her polyuria?
A. Primary polydipsia
B. Gestational diabetes mellitus with osmotic diuresis
C. Intracranial space occupying lesion
D. Vasopressinase secretion with underlying partial vasopressin disorder
What is (D)
Gestational diabetes insipidus occurs because of placental production of vasopressinase, which begins at week 7 of pregnancy and increases with placental growth. At peak levels, it is associated with 80%–85% reduction in circulating arginine vasopressin (AVP) levels, resulting in polyuria. Because of intact osmosensation, the patient perceives intense thirst sensation, leading to polydipsia. This is a rare complication that occurs in four patients per 100,000 pregnancies. Having an underlying partial vasopressin disorder (such as partial deficiency or resistance to vasopressin) may lead to symptomatic polyuria and even hypernatremia, when there is vasopressinase activity. Since the patient has mild hypernatremia despite drinking five to six bottles of water every day, primary polydipsia (answer A) is not the correct answer. Her daily osmolar excretion is 220 x 3.5, which equals 770 mOsm/d, which is typically within the limits of what is expected from a Western diet and is not suggestive of osmolar diuresis. Hence, although mild hyperglycemia leads to increased urination, the polyuria is not completely explained by this. Thus, answer B is incorrect. Having arginine vasopressin deficiency (AVP-D) from an intracranial space-occupying lesion (answer C) is possible; however, considering that she is in her third trimester of gestation, answer D is more likely.
A 26-year-old man is found to have IgA nephropathy on a renal biopsy performed for intermittent hematuria and
persistent proteinuria (1.8 g/d). His serum creatinine is 1.2 mg/dl. Urinary protein excretion declines to 1.5 g/d, and the serum creatinine increases to 1.3 mg/dl after 3 months of lisinopril 10 mg/d. The urinary Na1 excretion is 180 mmol/d.
Which ONE of the following should be done next?
A. Add 50 mg/d losartan
B. Increase lisinopril to 20 mg/d
C. Add 25 mg/d spironolactone
D. Start oral steroids at 1 mg/kg per d
E. Instruct on a low NaCl diet
What is (E)
Instruct on a low NaCl diet
The initial treatment of choice of IgA nephropathy is inhibition of angiotensin action by angiotensin converting enzyme inhibitors or angiotensin receptor blockers. However, the bene ts of these agents on proteinuria and outcome can be overcome by high NaCl diets. This patient is consuming a high NaCl diet as indicated by the urinary Na excretion. A low NaCl diet should be tried before changing the regimen.
A 35-year-old woman presents to the emergency department after a fall complicated by a fracture of her distal tibia. Her past medical history includes HIV and acne vulgaris. Her HIV has been well controlled with bictegravir, emtricitabine, and tenofovir for the last 2 years. On review of systems, she reports polyuria and polydipsia.
Laboratory data are as follows:
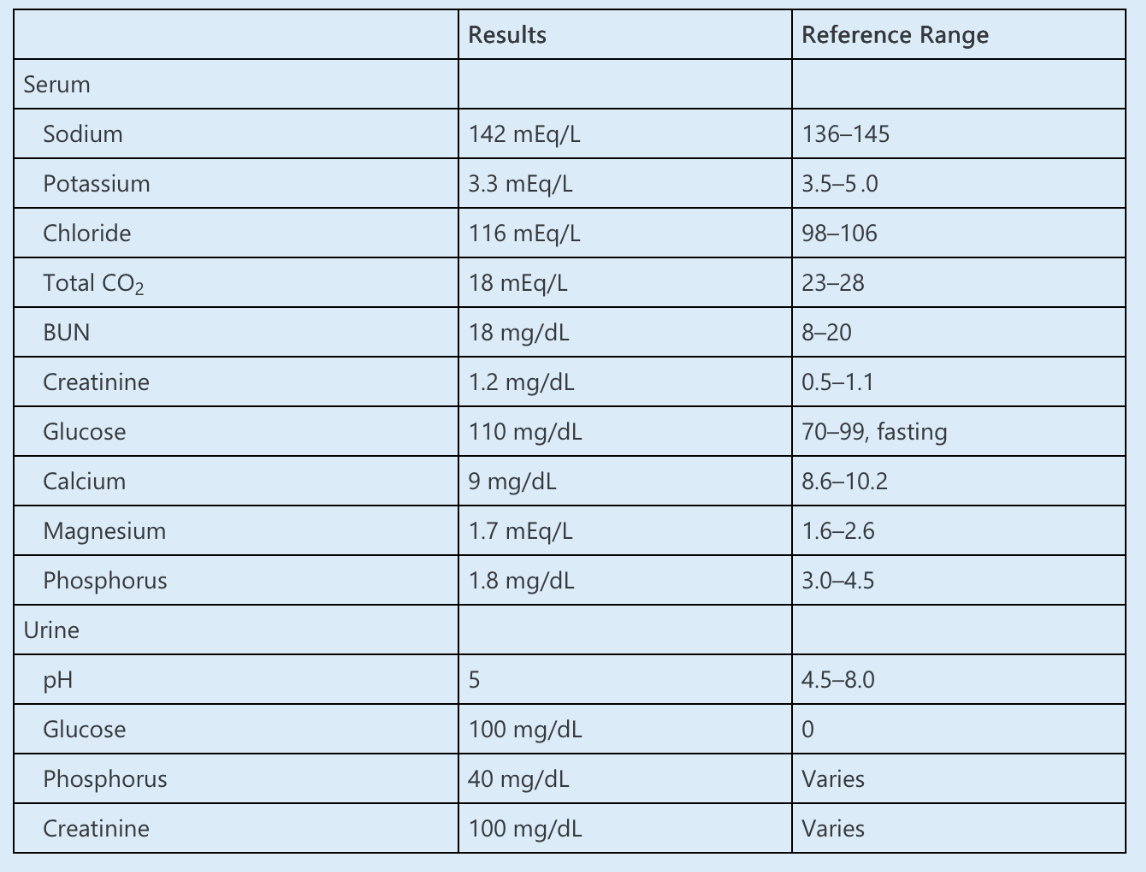
Which ONE of the following is the MOST likely diagnosis?
A. Fanconi syndrome
B. Diabetes mellitus
C. Distal renal tubular acidosis
D. Vitamin D deficiency
E. Hyperparathyroidism
What is Fanconi syndrome?
Fanconi syndrome, or a proximal tubulopathy, presents with numerous signs and symptoms of proximal tubular dysfunction. Patients with Fanconi syndrome typically have hypokalemia and hypophosphatemia from potassium and phosphorus wasting, respectively. Polyuria results from impaired sodium and water reabsorption. Loss of bicarbonate and glucose reabsorption in the proximal tubule result in a proximal renal tubular acidosis (RTA) and glucosuria despite euglycemia (note that the other common cause of glucosuria with euglycemia is the use of sodium-glucose cotransporter 2 inhibitors).
To categorize the renal response to hypophosphatemia, the fractional excretion of phosphorus (FEphos) can be calculated as follows:
FEphos = [phosphorus (urine) × creatinine (serum)] ÷ [phosphorus (serum) × creatinine (urine)] × 100%
FEphos = [40 × 1] ÷ [1.8 × 100] × 100% = 22%
FEphos greater than 5–20% suggests a renal etiology of hypophosphatemia.
Fanconi syndrome can be genetic or acquired. This patient has a history of long-standing tenofovir use, which can cause acquired Fanconi syndrome. Tubular toxicity is more common with tenofovir disoproxil fumarate (TDF) than tenofovir alafenamide, a prodrug that more efficiently delivers the active drug to its target cells, allowing for a lower dose and reduced side effects.
Although this patient has polyuria, polydipsia, and glucosuria, she has a random blood glucose level <200 mg/dL without symptoms, which is not consistent with a diagnosis of diabetes.
A type 1 (distal) RTA would present with hypokalemia and a non-anion gap metabolic acidosis, but renal phosphate or glucose wasting is not seen in distal RTA.
Your patient is a 59-year-old man with ESKD secondary to diabetes and hypertension who has been on in-center dialysis for 6 months. Medications include lisinopril 40 mg, carvedilol 25 mg twice a day, and amlodipine 10 mg every day. His dialysis sessions are often complicated by intradialytic hypotension. He is extremely compliant with medication and worries that, on dialysis days, his BP is higher than his usual target of 125/80 mm Hg. He tells you that before starting dialysis, his doctors stressed the importance of maintaining strict BP control to prevent “heart attacks, strokes, and kidney failure.”
Given current data, which of the following provides the best approach to predialysis BP targets in ESKD patients?
A. The predialysis BP target is no different than that for high cardiovascular risk patients who are not on dialysis
B. Predialysis BP should be lower than that for high cardiovascular risk patients who are not on dialysis
C. The predialysis BP target should be adjusted up in patients in order to prevent intradialytic hypotension.
D. The predialysis BP target should be <140/90 mm Hg as per the 2005 Kidney Disease: Improving Global Outcomes guidelines
What is (C)
The most recent guideline that defined a BP goal for dialysis patients is the 2005 Kidney Disease: Improving Global Outcomes guidelines, which recommended a predialysis BP goal of <140/90 mmHg. However, a subsequent study demonstrated that predialysis BP targets of <110–140 mmHg compared with higher targets of 155–165 mmHg increased the risk of intradialytic hypotension, which has been associated with increased cardiovascular and all-cause mortality. The lower predialysis target was also associated with higher postdialysis weights. A general current consensus is to prioritize achieving a target EDW and avoid intradialytic hypotension as much as possible. Answers A, B, and D are incorrect, because all would result in a predialysis target <140/90 mmHg.
A 68-year-old woman with a longstanding history of Sjögren disease and hypertension presented with persistent weakness and polyuria. She was taking amlodipine for hypertension. She also had some mild diarrhea in the past month. On examination, she was mildly dehydrated. Laboratory tests showed a serum sodium of 140 mEq/L, potassium 2.5 mEq/L, chloride 110 mEq/L, 152 bicarbonate 17 mEq/L, and creatinine of 1.1 mg/dl. The ABG was pH 7.32, pO2 95 mm Hg, pCO2 32 mm Hg, HCO3 16 mEq/L. Her urine pH was 6.5, urine sodium 78 mEq/L, urine potassium 30 mEq/L, urine chloride 68 mEq/L, urine urea nitrogen 800 mg/dl, and urine osmolarity 550 mOsm/kg. Urinalysis was negative for glucose, ketones, and protein.
What is the MOST likely cause of the patient’s hyperchloremic metabolic acidosis?
A. Distal (Type 1) RTA
B. Proximal (Type 2) RTA
C. Hyperkalemic (Type 4) RTA
D. Gastrointestinal bicarbonate loss due to diarrhea
What is Distal RTA?
The diagnosis of distal or type 1 RTA is supported by the patient’s history of Sjögren syndrome, which is often associated with distal RTA, and her laboratory results showing severe hypokalemia and an inappropriately high urine pH despite systemic acidosis.
Key in distinguishing distal RTA from other causes of hyperchloremic metabolic acidosis, such as gastrointestinal bicarbonate loss, is the assessment of urine ammonium excretion. In distal RTA, the inability to acidify the urine leads to a persistently high urine pH (>5.5) and low ammonium excretion. Because of the limited availability of urine ammonium excretion tests in most clinical laboratories, calculations of UAG and urine osmolal gap (UOG) provide surrogate markers. The UAG, calculated as urine sodium plus potassium minus chloride, is +40 mEq/L, in this case UAG = (78 + 30) − 68). A positive UAG >20 typically indicates low ammonium excretion, which is a hallmark of distal RTA. By contrast, gastrointestinal bicarbonate loss would present with a negative UAG due to increased ammonium excretion. Furthermore, the UOG, calculated as measured urine osmolality (550 mOsm/kg) minus calculated urine osmolality ([2 x (78 + 30)] + [800]/2.8 = 501 mOsm/kg), is 49 mOsm/kg. A UOG of less than 150 mOsm/kg in a patient with chronic metabolic acidosis suggests that ammonium excretion is impaired, consistent with distal RTA rather than gastrointestinal cause of acidosis.
A 56-year-old woman is found to have normochromic-normocytic anemia, hypophosphatemia, hypouricemia,
glycosuria, proteinuria (11 by dipstick testing), and renal insuf ciency (serum creatinine concentration of
2.6 mg/dl). Urine protein excretion was 3.1 g/d.
Which ONE of the following is the MOST LIKELY cause of this constellation of ndings?
A. Medullary cystic disease
B. Lead intoxication
C. Aristolochic acid intoxication
D. Multiple myeloma
E. Adult-onset cystinosis
What is (D) Multiple myeloma
This older woman has a normocytic, normochromic anemia, renal failure, heavy proteinuria, and all of the
features of adult-onset Fanconi syndrome. The most common cause of this constellation of ndings is multiple myeloma with tubular deposition of the monoclonal Ig within proximal tubule cells (often in the form of crystals), leading to proximal tubule dysfunction (Fanconi syndrome) (answer D). The heavy proteinuria is most likely a combination of tubular proteinuria and the excessive excretion of monoclonal light chains (typically k light chains) in the urine. Medullary cystic disease, lead intoxication, and aristolochic acid intoxication would not cause these ndings (answers A, B, and D). Adult-onset cystinosis could cause heavy proteinuria (due to FSGS), but Fanconi syndrome is much less likely and the patient is quite old for this diagnosis (answer E).
You have a 52-year-old male patient with CKD stage 2 and resistant hypertension, who is already taking valsartan 80 mg, chlorthalidone 100 mg, and amlodipine 10 mg. The patient also takes a statin to manage his high cholesterol, but he has no other comorbidities. BP remains uncontrolled. You are looking for medications with different mechanisms of action to try to achieve a synergistic effect.
What is the mechanism of action of aprocitentan?
A. Inhibits renin
B. Blocks β-adrenergic receptor 1
C. Inhibits endothelin receptor A
D. Inhibits both endothelin receptors A and B
What is (D)
Aprocitentan is the active metabolite of macitentan, a dual inhibitor of endothelin receptors A and B. Therefore, the correct answer is D, which also makes answer C incorrect. Answers A and B are wrong because these are the mechanisms of direct renin inhibitors and β-blockers, respectively.
You are asked to give a second opinion on a patient with resistant hypertension. Secondary causes have been excluded. He is on an ACEI, β-blocker, thiazide-type diuretic, and calcium blocker. He believes his quality of life is reduced by the number of medications he is taking, and he is unwilling to escalate doses or try additional medication. The patient inquires about a new technique called renal denervation (RDN).
Which of the following statements is correct regarding RDN?
A. RDN has not yet been approved for use in the United States
B. RDN is effective for patients regardless of eGFR
C. RDN is most effective for patients with low sympathetic activity
D. RDN candidacy should be evaluated with out-of-office BP measurements
What is (D)
Renal denervation (RDN) is a relatively new procedure-based method to treat resistant hypertension. The Food and Drug Administration has approved two RDN systems for use including the Paradise Ultrasound Renal Denervation System and Symplicity Spyral system, which use ultrasound and multielectrode radiofrequency, respectively, to ablate the nerves. RDN has been shown to be effective and safe in a number of trials; however, it has not been studied in individuals with eGFR <40 mL/min per 1.73 m2. RDN is most effective among patients with high sympathetic activity. Patients likely to respond have been characterized in a meta-analysis as having higher heart rate and lower pulse wave velocity.
The American Heart Association published a scientific statement to define patients amenable to RDN; it stresses that hypertension should be verified with out-of-office BP measurements, and those with secondary hypertension and renal artery abnormalities should be excluded.
In the absence of an alkali load, a patient with which defect can still present with metabolic alkalosis?
A. Pendrin mutation.
B. CFTR mutation.
C. Secretin receptor mutation.
D. Von Hippel Lindau gene mutation.
What is CFTR mutation
Mouse models using secretin receptor mutations and CFTR mutations suggest that secretin and CFTR help upregulate expression, apical localization, or function of pendrin. Any dysfunction in pendrin only manifests in the presence of an alkali load and not at baseline; thus answers A and C are incorrect. However, cystic fibrosis patients can have two mechanisms that can lead them to develop metabolic alkalosis, which is why they can present with either high or low urine chloride. CFTR regulation on pendrin and a pseudo-Bartter syndrome are those mechanisms. Pseudo-Bartter syndrome is often found in neonates with cystic fibrosis and results from loss of chloride from sweat, resulting in a secondary hyperaldosteronism and metabolic alkalosis independent of an alkali load. Answer D is incorrect. von Hippel Lindau gene mutation does not usually present with an acid-base abnormality and is characterized by hemangioblastomas of the brain, spinal cord, and retina; renal cysts and clear cell renal cell carcinoma; pheochromocytoma and paraganglioma; pancreatic cysts and neuroendocrine tumors; endolymphatic sac tumors; and epididymal and broad ligament cystadenomas.
A 36-year-old woman is found to have intermittent hematuria and proteinuria (21 by dipstick testing). There is no family history of renal disease. Her BP is 150/92 mmHg, and serum creatinine is 1.4 mg/dl. The urine protein/creatinine ratio is 1.2 g/g. Serum albumin and C3 complement are normal; the antinuclear antibody level is 1:40.
Which ONE of the following is the MOST likely to be present on further study?
A. Elevated serum levels of undergalactosylated IgA1
B. Reduced serum levels of C4
C. Decreased ratio of IgA to C3 in serum
D. Elevated serum levels of monomeric IgA
E.Elevated serum levels of C-reactive protein
What is (A) Elevated levels of undergalactosylated IgA1
The clinical features strongly suggest a diagnosis of primary (sporadic) IgA nephropathy. Of the possible
choices, only increased levels of IgA1 having abnormally low levels of galactose side-chains on the hinge region of IgA1 have been associated with primary IgA nephropathy on a consistent basis. C4 hypocomplementemia is not seen in primary IgA nephropathy and the ratio of IgA to C3 in serum is increased not decreased in IgA nephropathy. Total IgA levels can be elevated in approximately 50% of patients with IgA nephropathy, but these primarily consist of polymeric not monomeric IgA. C-reactive protein levels are not increased in IgA nephropathy.
A 37-year-old woman presents to the emergency department with 3 months of generalized weakness, muscle aches, cramping, and increasingly dark urine. Symptoms have worsened over the last 2 weeks, during which time the patient began training for a marathon, running up to 6 miles daily and increasing her water and sports drink intake. Her past medical history includes hyperlipidemia and Sjögren syndrome. Her medications include simvastatin and hydroxychloroquine.
On examination, her BP is 132/80 mm Hg, her heart rate is 76/min, and her respiratory rate is 18/min. She is afebrile. Other than dry oral mucosa and some deep tenderness of the large muscles, her examination is unremarkable.
Laboratory data are as follows:
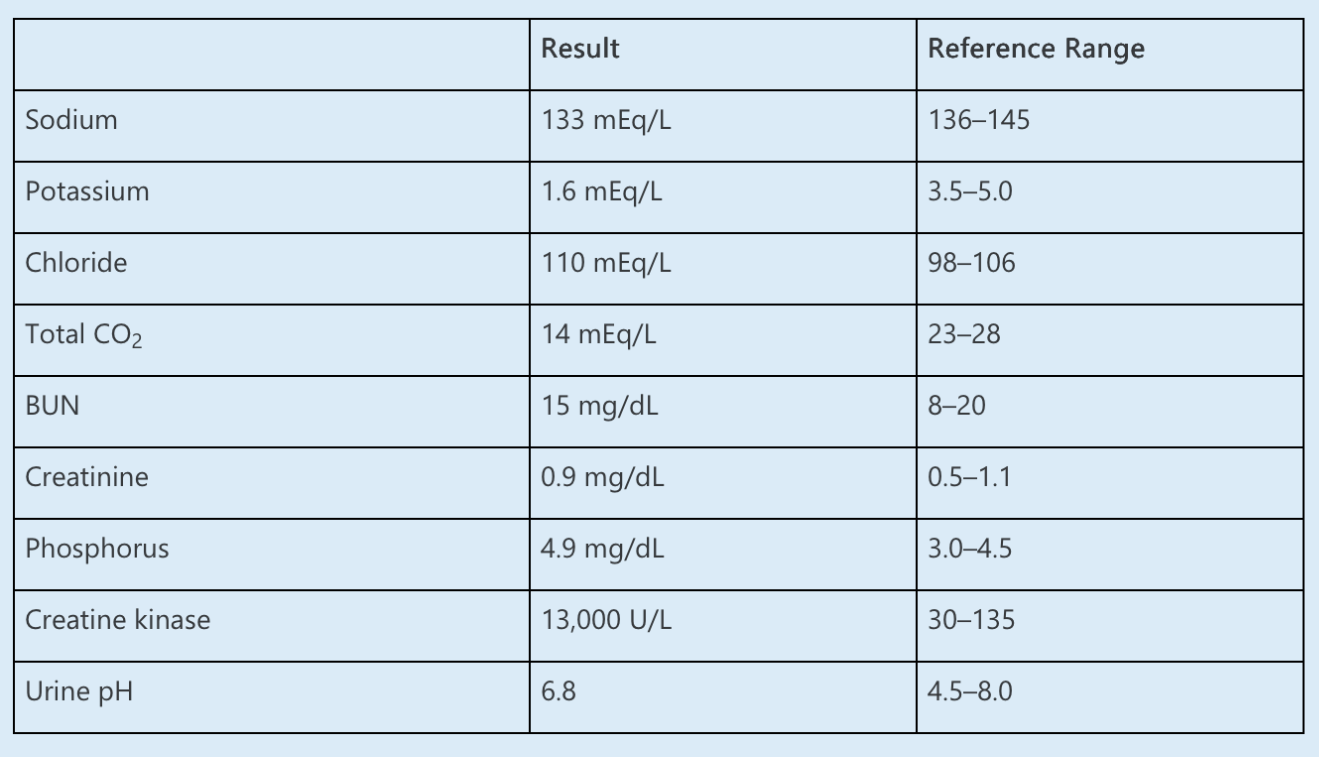
Which ONE of the following is the most likely diagnosis?
A. Distal renal tubular acidosis
B. Excessive exercise
C. Hypokalemic periodic paralysis
D. Simvastatin toxicity
E. Surreptitious diuretic use
What is Distal renal tubular acidosis
In this young patient with Sjögren syndrome, marked hypokalemia, and a non–anion gap metabolic acidosis, the most likely diagnosis is rhabdomyolysis attributable to hypokalemia induced by her distal (type 1) renal tubular acidosis (RTA).
Some patients with Sjögren syndrome (up to 25%) have been found to lack the H+ ATPase on the luminal membrane of the α-intercalated cells of the distal nephron. Some patients may also generate antibodies against carbonic anhydrase in these cells, preventing generation of H+ and regeneration of HCO3-. These defects lead to both impaired hydrogen ion secretion and potassium reabsorption. The resulting metabolic acidosis also leads to increased distal tubular sodium delivery, as the decreased filtered load of bicarbonate leads to less sodium-bicarbonate reabsorption in the proximal tubule with increased distal sodium delivery, further enhancing urinary potassium losses. Urine pH is relatively alkaline due to impaired distal H+ secretion. Severe hypokalemia, occasionally with paralysis and respiratory arrest, has been reported as the initial presentation in some cases of Sjögren syndrome. Normally, relative hyperkalemia in exercising muscle leads to the release of vasodilatory factors and increased blood flow. In hypokalemia, this response is impaired, which can lead to skeletal muscle ischemia and myocyte necrosis, and ultimately rhabdomyolysis.
Your patient has been on single-agent antihypertensive therapy for 3 months and has still not reached target BP despite now being at the maximum dose. You plan to add another agent and would like the patient to check home BP readings to evaluate response.
How many measurements per day should the patient perform?
A. One measurement (in the morning)
B. Two measurements (one in the morning, one in the evening)
C. Three measurements (one in the morning, one at midday, one in the evening)
D. Four measurements (two measurements in the morning separated by 1 minute, and two measurements in the evening separated by 1 minute)
What is (D) Four measurement
The most widely used and validated frequency of home BP measurement uses the “722 protocol,” with patients obtaining a total of four measurements during the day:
two in the morning before medication and two in the evening. Averaging BP readings obtained using the “722” produces values that correlate strongly with readings obtained via ABPM.
A 35-year-old man with a past medical history of endstage renal disease on hemodialysis is admitted to the neurosurgical intensive care unit after a motor vehicle crash. Computed tomography scan of the head shows evidence of right-sided subdural hematoma and cerebral edema with 3-mm midline shift. He is also hyperkalemic to a potassium concentration of 6.4 mEq/L. Nephrology is consulted, and a decision is made to initiate continuous venovenous hemofiltration (CVVH) while also maintaining permissive hypernatremia to 150 mEq/L through 3% hypertonic saline infusion through a central venous catheter. The calculated replacement fluid rate is 2200 ml/h, which will be administered prefilter, and the blood flow rate is 230 ml/min with no net ultrafiltration. His current plasma sodium is 140 mEq/L, and the sodium concentration in the replacement fluid is also 140 mEq/L.
While starting CVVH, which of the following is the recommended infusion rate for the 3% hypertonic saline?
A. 30 ml/h
B. 60 ml/h
C. 75 ml/h
D. 100 ml/h
60ml/hr
This patient needs renal replacement therapy to control his hyperkalemia,
because he has end-stage renal disease (ESRD). Because of increased intracranial pressure from
the subdural hematoma and cerebral edema, it is unsafe to perform intermittent hemodialysis,
because osmolar fluid shifts may potentially worsen brain edema, increasing the risk of
herniation. Hence, continuous venovenous hemofiltration will be performed to minimize rapid
osmolar changes in the plasma from urea removal. To maintain permissive hypernatremia, 3%
hypertonic saline solution is infused. To maintain a plasma sodium concentration of 150 mEq/L,
the following formula can be used to calculate the 3% saline infusion rate:

estimated 3% saline infusion rate = (150 − 140) (2200)/(513 − 150) ≈ 60 ml/min
where QD = quantity of dialysate fluid = 0 ml/h
QRF = quantity of replacement fluid = 2200 ml/h
Which ONE of the following clinical descriptions is MOST compatible with the renal biopsy shown in
Figure 1?
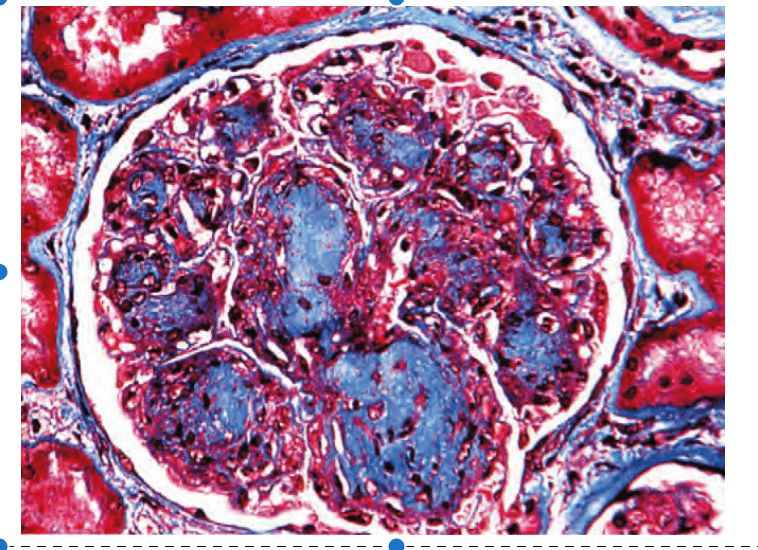
Figure 1. Mallory trichrome stain.
A. A 68-year-old Caucasian woman with a lifelong history of heavy smoking and no history of abnormal glucose metabolism
B. A 24-year-old woman with a family history of lupus erythematosus and repeated miscarriages and no history of abnormal glucose metabolism
C. A 16-year-old boy with pulmonary hemorrhage and ARF but no history of abnormal glucose metabolism
D. A 29-year-old African-American man with sickle cell disease and no history of abnormal glucose metabolism
E. A 63-year-old man with postural hypotension, easy bruising, hepatomegaly, and nephrotic syndrome and no history of abnormal glucose metabolism
What is (A)
A 68-year-old Caucasian woman with a lifelong history of heavy smoking and no history of abnormal glucose metabolism.
The glomerular lesion illustrated is that of nodular intercapillary glomerulosclerosis (the Kimmelstiel–Wilson lesion). Although this lesion is most often seen in patients with diabetic nephropathy, none of the choices offered have any evidence of glucose dysmetabolism. Among the choices offered, only“idiopathic” nodular glomerulosclerosis is a reasonable possibility (answer A). This newly described entity is seen most typically in older women with a strong history of excessive and long-term smoking. The lesion illustrated is not amyloidosis, sickle cell–associated membranoproliferative GN, lupus nephritis, with anti-phospholipid syndrome, or Goodpasture’s disease (answers B through E).
A 65-year-old woman with a history of breast cancer metastatic to the liver presents with 2 weeks of shortness of breath, fatigue, anorexia, weight loss, and back pain. She has received zoledronic acid monthly in an attempt to improve bone pain and prevent fractures.
BP is 130/80 mm Hg, heart rate is 82/min, and oxygen saturation is 98% while breathing room air. Physical examination is significant for dry oral mucosa, clear lungs, and no peripheral edema.
Laboratory data are as follows:
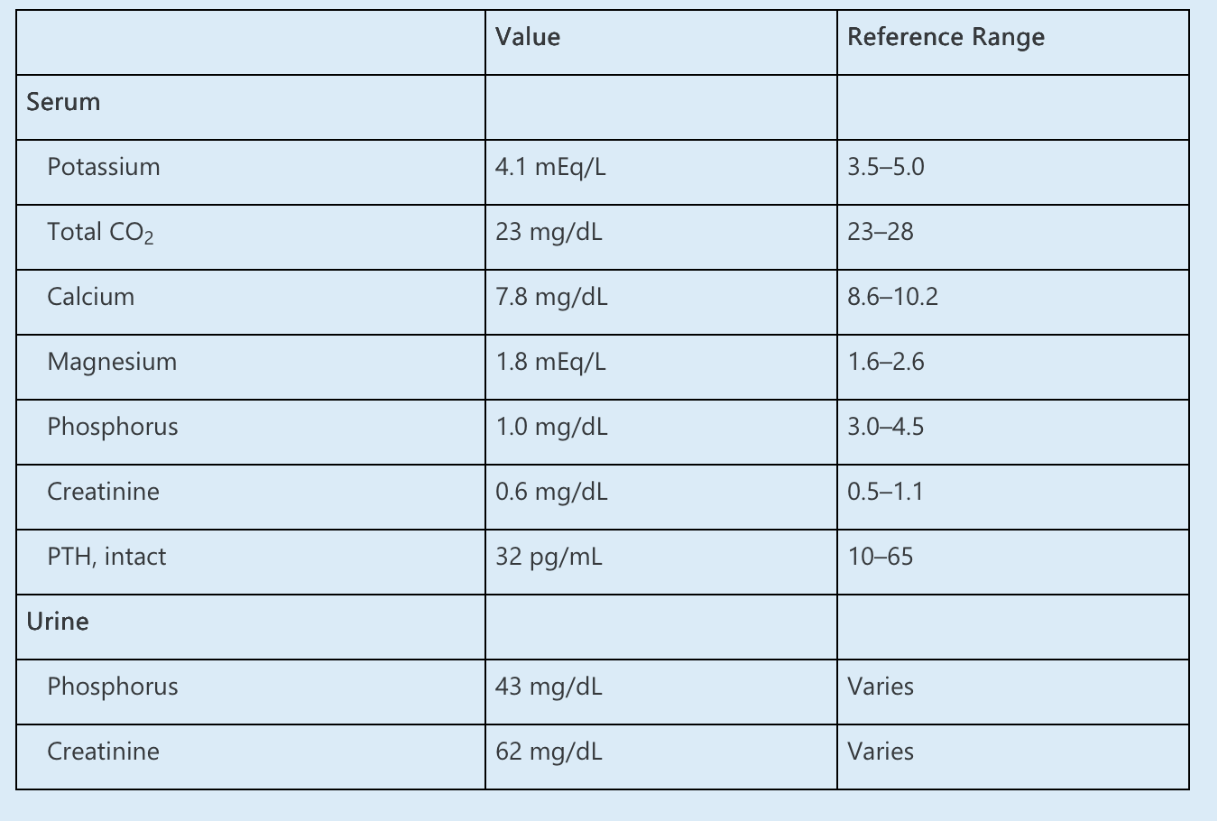
Which of the following is the MOST LIKELY cause of hypophosphatemia?
A. Falsely low phosphorus measurement caused by interference of tumor-related proteins
B. Urinary phosphorus wasting from proximal tubulopathy
C. Increased production of fibroblast growth factor-23 (FGF-23)
D. Decreased dietary phosphorus intake
E. Bisphosphonate-mediated calcium and phosphorus deposition in bone
What is increased production of FGF23
The initial evaluation of hypophosphatemia requires assessment of the renal response. The urine phosphorus and creatinine may be used to calculate a tubular reabsorption of phosphorus (TRP) or fractional excretion of phosphorus (FEphos), similar to the fractional excretion of sodium. In the setting of hypophosphatemia, TRP <80% or FEphos greater than 5–20% suggests inappropriate urinary phosphate wasting. In this case:
FEphos = [(urine phosphorus × serum creatinine) ÷ (serum phosphorus × urine creatinine)] × 100%
= (43 × 0.6) ÷ (1.0 × 62) × 100%
= 41%
Thus, the low serum phosphorus is likely attributable to renal losses rather than inadequate dietary intake or bisphosphonate-mediated bone deposition.
TIO is a well-recognized oncologic cause of urinary phosphate wasting and has been reported with sarcomas and breast and colon carcinomas. TIO is caused by tumoral overproduction of FGF-23, a phosphatonin that causes internalization of tubular sodium-phosphate cotransporters and inhibits calcitriol production by 1α-hydroxylase. Although hypophosphatemia is the predominant manifestation, hypocalcemia and elevated PTH levels may also occur. Tumor tissue staining for FGF-23 and high plasma levels of FGF-23 are consistent with a diagnosis of TIO. TIO is associated with an increased risk of bone fractures and mortality, so early and aggressive supplementation of phosphorus, calcium, and activated vitamin D may be required, as well as treatment of the underlying malignancy.