What are elements trying to achieve when chemically bonding?
All atoms trying to achieve a stable octet (they want 8 electrons in their valance shell)
How to draw a Lewis Dot Structure for ionic compounds?
Determine which atom will be the +ion
Determine which atom will be the - io
Write the symbol for the + ion first.
NO DOTS
Draw the e- dot diagram for the – ion
COMPLETE outer shell
Enclose both in brackets and show each charge
How do you name a covalent compound?
add a prefix to both elements based on the subscript
How do you write a chemical formula for an ionic compound?
1) Write the symbols for the ions.
2) Add the charge to each ion. How many electrons will each ion lose or gain?
3) Cross the charges to balance the charges between ions.
4) Check the combined positive and negative charges to see if they cancel out.
5) Remove the charges. Simplify subscripts to simplest whole-number ratio. Put them together.
What is the main difference between binary acids and oxyacids?
Binary acids are produced with a monoatomic anion and oxyacids are produced with a polyatomic ion.
Properties of Ionic Compounds are...
Hard solids at 22C
High melting point temperatures
Nonconductors of electricity in solid phase
Good conductors in liquid phase or dissolved in water (aq)
How to draw a Lewis Dot Structure for covalent compounds?
Determine the type and number of atoms.
Write the Lewis Dot Structure for each type of atom.
Determine the total number of valence electrons available for bonding.
Arrange the atoms to form a skeleton structure for the molecule.
Add unshared electrons to each nonmetal atom so that it has a full octet (except hydrogen).
How do you name an ionic compound?
list the metal first
change the ending of the anion to "-ide"
How do you write a chemical formula for a covalent compound?
The first half of the name will give you the first element symbol and its subscript.
If there is no prefix, then there is only one atom for that element (and we don’t write the number one).
The second half of the name will give you the second element symbol and its subscript.
What changes do you need to make when naming a binary acid?
- add the prefix hydro-
- replace ending of anion with "-ic"
- add the word acid
Properties of Covalent Compounds are...
Low melting point and boiling point temperatures
Relatively soft solids as compared to ionic compounds
Nonconductors of electricity in any phase
Draw the Lewis Dot Structure for NaCl

When naming ionic compounds with transition metals you need to include roman numerals to show the _____ of the metal.
charge
What is the formula of carbon tetrachloride?
CCl4
What changes do you need to make when naming an oxyacid?
replace the ending of polyatomic ion
- "ic" if anion ends with "ate"
- "ous" if anion ends in "ite"
add acid to the end
Properties of Metallic Compounds are...
High melting point temperatures
Ductile, malleable, shiny
Hard substances
Good conductors of heat and electricity
What element is this?

PCl3
Name CF4
Carbon tetrafluoride
What is the formula of calcium phosphate?
Ca3(PO4)2
Name HCI
hydrochloric acid
What elements don't follow the octet rule?
Hydrogen (happy with two electrons)
Boron (happy with six electrons)
Main-group elements in Periods 3 and up can form bonds with expanded valence, involving more than eight electrons.
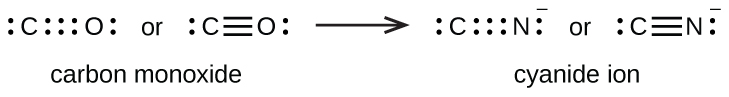
Draw the Lewis Dot Structure for CO
The name of FeCl₂ is
iron (II) chloride
What is the formula for manganese(III) oxide?
Mn2O3
Name HNO3
Nitric acid