How many sigfigs are in the following number?
0.001030
4
What is the equation for density?
D = m/v
In the equation below, which variable represents specific heat capacity?
q=mcΔT
c
A particle with an equal number of protons and electrons is called a(n) _____________.
atom
A particle with an unequal number of protons and electrons is called a(n) _____________.
Ion
How many sigfigs are in the following number?
708090
5
What are the two possible options for units of volume that we learned about?
mL and cm3
What is the unit for specific heat capacity?
J/(g⋅°C)
12
What element has a ground state electron configuration ending in 4d9?
Silver (Ag)
How many sigfigs are in the following number?
708090.00
8
Which of the following (A, B, C, or D) is most dense?
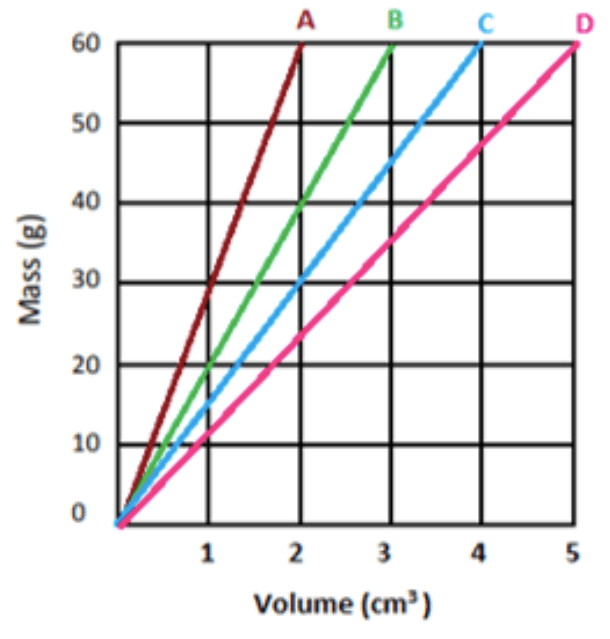
A
Which equation would you use at point B?
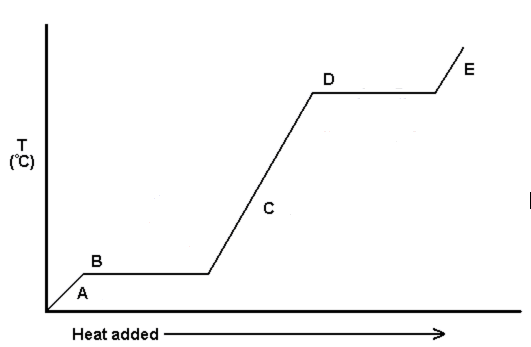
q = m ⋅ Hf
What is most likely to be the most abundant isotope of Argon?
Argon-40
Which of the following has the greatest force of attraction between electron and proton(s)?
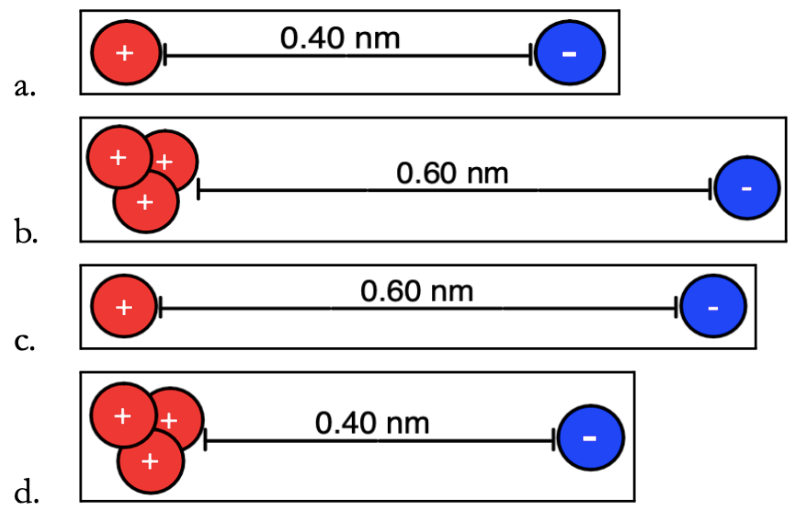
D
Write the following number in scientific notation?
709,000,000
7.09 x 108
What is the density of object D?

12 g/cm3
When 435J of heat is added to 3.4g of olive oil at 21°C, the temperature increases to 85°C. What is the specific heat of olive oil? (sigfigs and units count in your final answer)
2.0 J/g⋅°C
A Bi-3 ion has how many protons?
83
An electron moves from energy level 5 (n=5) to energy level 4 (n=4). The electromagnetic radiation would have a _______ wavelength and ______ energy.
1. Long
2. Low
There are 149,600,000 kilometers between the Earth and the Sun. How many miles is this? (Hint: there are 1.609 km in 1 mile)
Yes sigfigs and units count in your final answer!
92,980,000 miles
An object has a volume of 6.25 mL and a density of 3.04 g/mL. Calculate the mass. (Include the correct number of sigfigs and units)
m = 19.0g
Calculate the energy required to melt 25.0 g of ice. (Hint: Hf = 334 J/g)
8,350 J
Write the atomic symbol for a bromine ion with one more electron than protons and 45 neutrons.
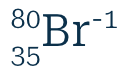
The force of attraction between the electron and the proton shown below is 6.0 N.

If the distance were to change to 0.10 nm, what would be the resulting force of attraction (include units)?
24 N