A process that changes one substance in to one or more different substances.
What is a chemical reaction?
Any push or pull on an object.
What is force?
The properties of the elements vary in a periodic way with their atomic numbers.
What is periodic law?
The energy that objects possess due to their motion.
What is kinetic energy?
What is mechanics?
A combinations of chemical formulas and symbols that models a chemical reaction.
What is a chemical equation?
A sketch that shows the object and the forces acting on it as vectors.
What is a free-body diagram?
DAILY DOUBLE
A table of the chemical elements arranged in a way that displays their periodic properties in a relationship to their atomic numbers.
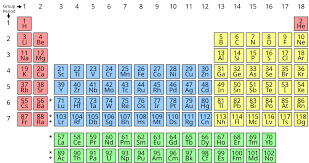
What is the periodic table?
Stored energy that can be used later.
What is potential energy
Occurs when an object's speed changes.
What is acceleration?
Substances that enter into a chemical reaction.
What are reactants?
The tendency of matter to resist change in its motion.
What is inertia?
Electrons in the outermost energy level of a neutral atom.
What is valence electrons?
Sum of any kinetic energy and all the forms of potential energy in a system.
What is mechanical energy?
The distance travelled in a amount of time.
What is speed?
Substances that reactants combine to produce.
What are products?
A pulling force that is transmitted through a rope, chain, or similar object.
What is tension?
A drawing that consists of the element's chemical symbol surrounded by the atom's valence electrons.
What is electron dot notation?
Rate at which an objects position changes.
What is velocity?
Any instance that electons are lost.
What is oxidation?
A feild force that acts between any two objects.
Measure of elements ability to attract and hold electrons when bonded to other atoms.
What is electronegativity?
Electrical energy generated by the movement of water.
What is hydroelectric energy?
DAILY DOUBLE
Velocity multiplied by its mass, uses the formula p=mv.
What is momentum?