What is chemistry the study of?
Matter & Change
What is the smallest particle of an element that retains its identity in a chemical reaction?
Atom
In 4th century BCE Democritus suggested matter is made up of tiny particles that can't be divided that he called _____.
In 4th century BCE Democritus suggested matter is made up of tiny particles that can't be divided that he called atoms.
All atoms of the same element contain the same number of what?
Protons
The sum of the number of neutrons and protons in an atomic nucleus
Mass Number
What are matter & energy?
Matter - Anything that takes up space
Energy - The ability to do work or produce heat
What are the 3 subatomic particles that make up an atom? What are their relative masses & charges
Proton - 1amu & positive charge (+1)
Neutron - 1amu & neutral charge (0)
Electron - ~0amu & negative charge (-1)
Rutherford discovered in 1911 through his famous gold foil experiment that atoms are mostly _______ & that the majority of their mass is located in a small central region called the _____.
Rutherford discovered in 1911 through his famous gold foil experiment that atoms are mostly empty space & that the majority of their mass is located in a small central region called the nucleus.
Atoms of the same element but with different mass numbers are called?
Isotopes
The average mass of all the isotopes of an element
Atomic Mass
Give 3 examples of physical properties -
Color, length, volume, mass, opacity, density, hardness, luster, malleable, melting point, boiling point, etc.
How do you get the mass number for an atom? Where is the majority of the mass located in an atom?
Mass number = (# of protons) + (# of neutrons)
Nucleus
J.J. Thomson & Robert Millikan discovered that _______ are a fundamental part of all atoms & that they have a _______ charge.
J.J. Thomson & Robert Millikan discovered that electrons are a fundamental part of all atoms & that they have a negative charge.
Convert the following percent abundances to relative abundance
51.02%
8.11%
0.56%
.5102
.0811
.0056
A substance made up of atoms of two or more different elements joined by chemical bonds
Compound
Give 3 examples of chemical properties -
Acidity, reactivity, flammability, toxicity, radioactivity, corrosiveness, PH, etc.
What is the atomic number & atomic mass of Uranium?
Atomic Number - 92
Atomic Mass - 238.03
Which scientist is credited with discovering that all elements are composed of tiny, indivisible particles called atoms & that an element is composed of a single type of atom?
John Dalton
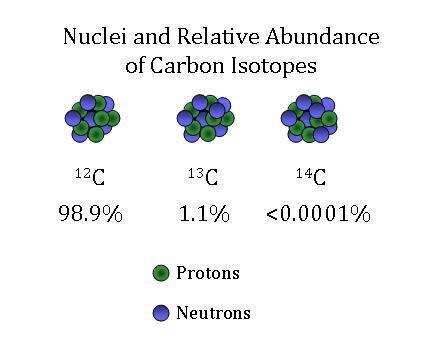
Carbon has 3 isotopes, Carbon-12, Carbon-13, & Carbon-14. Given that Carbons atomic mass is 12.011, which carbon isotope has the highest abundance, how do you know?

Carbon-12 has the highest abundance since its mass number 12 is closest to carbons atomic mass 12.011
The single capital letter or capital letter followed by a lower case letter or letters that represents the name of the element.
Element Symbol
A student mixes baking soda & vinegar, it quickly starts to react creating bubbles, giving off carbon dioxide gas, & heating up. Is this an example of a physical or chemical change? Give 2 reasons you know this -
Chemical change, because it reacts to create bubbles, gives of carbon dioxide gas, & releases its own heat.
How many of each subatomic particle are located in neutral Platinum-195?
Protons - 78
Neutrons - 117
Electrons - 78
This model of the atom was created in 1913 & showed that electrons orbit around the nucleus based on their energy level. Often called the planetary model.
 Bohr model
Bohr model
You are given a sample of a mystery element, the element has 2 isotopes.
Element-79 is 50.7% abundant
Element-81 is 49.3% abundant
Calculate the atomic mass of the mystery element & use the periodic table to determine its identity.
79.986 amu - Bromine
An elemental symbol with a superscript that indicates the mass number and a subscript that indicates the atomic number for a specific isotope
Nuclear Symbol