What is the rate law of this elementary reaction step?
A+3B->2C
r=k [A][B]3
What are the units of k for a first order reaction?
1/s
Graphed concentration vs time. Hat order is this?
1st
For a zero order reaction, rate=_____
k
The rate of a reaction with just two reactants is observed to double when the concentration of one reactant is doubled and the second reactant is held constant. The rate is also observed to increase by a factor of nine when the
concentration of the second reactant. is tripled, holding the concentration of the first reactant constant. What is the overall order for this reaction?
3rd order
What is the rate law of this reaction?
A+B->2C. Fast
2B+ D->C. Slow
r=k[B]2[D]
What are the units of k for a second order reaction?
1/M*s
Graphed concentration vs time. what order is this?
2nd
If I have a first order reaction, and R=k[A], then 2A=___?
2k[A]
When reacted with water, insecticides DT decomposes with a half-life of 10 years. Approximately how many years will it take for 9% of a given sample to decompose once exposed to water in the environment?
70 years
What is overall reaction order of this rate law?
A+B->2C. Fast
2B+ D->C. Slow
3rd overall order
What are the units of K for a zero order reaction?
M/s
At hat point is the intermediate formed?
I
What is the integrated rate law of a first order reaction?
ln[A]=ln[A]0-kt
At a certain temperature the first-order decomposition of hydrogen peroxide exhibits these data points
Time(s) [H2O2](1/M)
0 2.0
15 1.0
At what time will [H2O2]=0.50 1/M
30 s
A certain reaction has a H=-75kJ and an activation energy of 40kJ. A catalyst is found that lowers the activation energy of the forward reaction by 15kJ. What is the activation energy of the revers reaction in the presence of this catalyst?
100kJ
What conditions affect the value of k?
Increase or decrease of temperature
Which is the slow step? (the first or second bump)
second
What is the half life equation of a zero order reaction?
t1/2=[A]0/2k
The Reaction 3O2->2O3 is proceeding with a rate of disappearance of O2 equal to 0.60 M/s. What is the rate of appearance of O3 in M/s?
0.40
A reaction and its rate law are given below. When [C4H6] = 2.0 M, the rate is 0.106 M/s. What is the rate when [C4H6] = 4.0 М? 2C4H6 →C8H12
Rate = k[C4H6]2
0.424 M/s
For a zero order reaction, the concentration of the reactants is doubled. What does the rate equal?
rate=k
Determine the average rate from 20-40s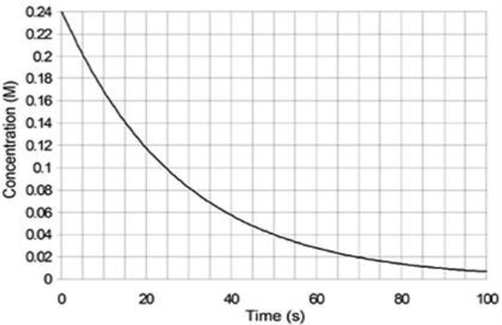
0.24M/s
What is the integrated rate law of a second order reaction?
1/[A]=1/[A]0+kt
An aqueous solution of H202 that is 6.00% H202 by mass, and has a density of 1.03 g/mL. Calculate the original number of moles of H2O2 in a 125 mL sample of the 6.00 percent H202 solution?
1.2 * 10-4M