This order reaction has a half-life equation of [A]i/2k.
What is 0 order?
True or False: Instant rate laws are always concerned with the reactants and not the products.
What is TRUE?
The order of reaction is determined by graphing data and seeing which graph is _______.
What is linear?
The energy threshold needed to have a reaction. The minimum amount of energy that must be present.
What is the activation energy?
This value is included in every one of the half-life equations for kinetics.
What is k (rate constant)?
This is the rate law for the following first order reaction:
A --> B
This order reaction is the only reaction that does not have a negative slope.
What is Second Order?
Collisions require these two factors to occur.
What are correct orientation and sufficient energy?
This is the half life equation of a second order reaction.
What is 1/k[A]i?
This rate order results in a rate that is independent of concentration.
What is 0 order?
A first order reaction has a linear curve on the graph ______ vs time.
What is ln[A]?
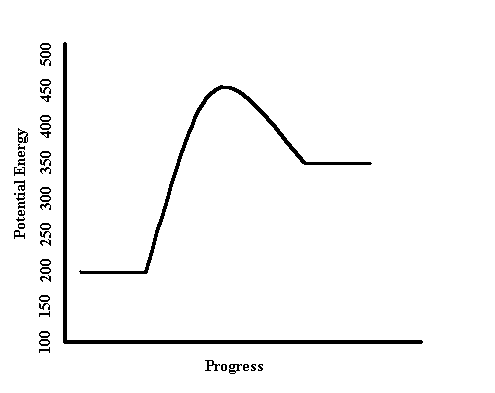 Endothermic or Exothermic.
Endothermic or Exothermic.
What is Endothermic?
True or False: all half life equations depend on the initial concentration.
What is FALSE?
Rate = k[A]3[B]0[C]1[D]1
What is 5?
The y intercept of a second order reaction.
What is 1/[A]i?
This is the molecule in a reaction sequence that is extremely high energy, and thus unstable. It occurs for only a brief amount of time.
What is the activated complex?
In first order half life, the number .693 comes from the natural log of this number.
What is 1/2?
Determine the rate law:
Experiment [NO] [Cl2] Rate (mol/L·s)
1 .250 .250 1.43 x 10-6
2 .500 .250 5.72 x 10-6
3 .250 .500 2.86 x 10-6
What is Rate = k[NO]2[Cl2]1?
First order reaction with initial concentration of .368M, 300 seconds pass, concentration of .0183M.
Determine k.
What is .01?
The length of the LHC (Large Hadron Collider).
What is 27 kilometers?