Which of the following describes an individual with two different alleles for a particular gene?
A. Homozygous dominant
B. Homozygous recessive
C. Heterozygous
D. Phenotypic
C. Heterozygous
Which gas makes up approximately 78% of the Earth's atmosphere?
A. Oxygen
B. Carbon dioxide
C. Nitrogen
D. Argon
C. Nitrogen
Which term refers to the process where organisms that are better adapted to their environment tend to survive and produce more offspring?
A. Genetic drift
B. Mutation
C. Natural selection
D. Speciation
C. Natural selection
Which type of radiation consists of a helium nucleus (2 protons and 2 neutrons)?
A. Alpha
B. Beta
C. Gamma
D. X-ray
A. Alpha
Calculate the mean of the following set of data: 12, 15, 18, 10, 20.
A. 15
B. 16
C. 17
D. 18
A. 15
What is the relative formula mass (Mr) of H2O? (Atomic masses: H=1, O=16)
A. 17
B. 18
C. 19
D. 20
B. 18
Which of the following is the correct unit for work done?
A. Watt (W)
B. Newton (N)
C. Joule (J)
D. Pascal (Pa)
C. Joule (J)
Which of the following is a primary product of photosynthesis?
A. Carbon dioxide
B. Water
C. Glucose
D. Oxygen (as a waste product)
C. Glucose
Which of the following units is used to measure electric current?
A. Volt (V)
B. Ohm (Ω)
C. Ampere (A)
D. Watt (W)
C. Ampere (A)
A reaction that releases energy to the surroundings, causing the temperature to increase, is called:
A. Endothermic
B. Exothermic
C. Catalytic
D. Reversible
B. Exothermic
Which term describes a community of living organisms interacting with their non-living environment?
A. Population
B. Habitat
C. Ecosystem
D. Species
C. Ecosystem
A substance with a pH value of 2 is considered to be:
A. Strongly alkaline
B. Weakly alkaline
C. Neutral
D. Strongly acidic
D. Strongly acidic
What is the observable characteristic or trait of an organism, resulting from the expression of its genes? A. Genotype
B. Phenotype
C. Allele
D. Chromosome
Phenotype
The process by which heat is transferred through the movement of fluids (liquids or gases) is known as:
A. Conduction
B. Radiation
C. Convection
D. Evaporation
C. Convection
Which of the following is considered strong evidence for evolution?
A. Lack of vestigial structures in modern organisms.
B. Identical DNA sequences across all species.
C. The fossil record showing transitional forms.
D. Absence of homologous structures among different species.
C. The fossil record showing transitional forms.
Which of the following statements is true regarding the penetration power of different types of radiation?
A. Beta particles are more penetrating than gamma rays.
B. Alpha particles are stopped by a sheet of paper.
C. Gamma rays can be stopped by a thin sheet of aluminium.
D. All three types of radiation have equal penetrating power.
B. Alpha particles are stopped by a sheet of paper.
Express 0.0000045 in standard form.
A. 4.5×106
B. 4.5×10−6
C. 4.5×105
D. 4.5×10−5
B. 4.5×10−6
How many moles are there in 44g of CO2? (Atomic masses: C=12, O=16)
A. 0.5 mol
B. 1.0 mol
C. 1.5 mol
D. 2.0 mol
B. 1.0 mol
A force of 50 N pushes an object a distance of 10 m in the direction of the force. How much work is done?
A. 500 J
B. 50 J
C. 5000 J
D. 5 J
A. 500 J
The small pores on the underside of leaves through which gases enter and leave are called:
A. Xylem
B. Phloem
C. Chloroplasts
D. Stomata
D. Stomata
According to Ohm's Law, if the voltage across a resistor is 12 V and the current flowing through it is 2 A, what is the resistance of the resistor?
A. 24 Ω
B. 6 Ω
C. 10 Ω
D. 4 Ω
B. 6 Ω
Which of the following is an example of an endothermic reaction?
A. Combustion of methane
B. Neutralisation of an acid and alkali
C. Photosynthesis
D. Respiration
C. Photosynthesis
Which of the following correctly lists the trophic levels in a food chain in order?
A. Producer → Primary Consumer → Secondary Consumer → Tertiary Consumer
B. Primary Consumer → Producer → Secondary Consumer → Tertiary Consumer
C. Producer → Secondary Consumer → Primary Consumer → Tertiary Consumer
D. Primary Consumer → Secondary Consumer → Tertiary Consumer → Producer
A. Producer → Primary Consumer → Secondary Consumer → Tertiary Consumer
Which of the following is a product of the reaction between a metal and an acid?
A. Salt and water
B. Salt and hydrogen gas
C. Salt and oxygen gas
D. Water and carbon dioxide
B. Salt and hydrogen gas
A genetic cross involves a dominant allele (A) for red flowers and a recessive allele (a) for white flowers. If a heterozygous red-flowered plant is self-pollinated, what is the probability of producing a white-flowered plant?
A. 0.75
B. 0.50
C. 0.25
D. 0.00
C. 0.25
Which of the following describes a homologous series? A. A group of compounds with different functional groups.
B. A series of compounds with the same general formula and similar chemical properties.
C. Compounds that are isomers of each other.
D. Elements with similar physical properties.
B. A series of compounds with the same general formula and similar chemical properties.
Antibiotic resistance in bacteria is a classic example of:
A. Punctuated equilibrium
B. Convergent evolution
C. Stabilizing selection
D. Natural selection
D. Natural selection
A radioactive isotope has a half-life of 6 hours. If you start with 160g of the isotope, how much will remain after 18 hours?
A. 80g
B. 40g
C. 20g
D. 10g
C. 20g
A student measures the mass of a substance as 25.4 g and its volume as 5.2 cm³. Calculate the density, giving your answer to an appropriate number of significant figures.
A. 4.8846 g/cm³
B. 4.9 g/cm³
C. 4.88 g/cm³
D. 5 g/cm³
B. 4.9 g/cm³
What mass of magnesium oxide (MgO) is produced when 24g of magnesium (Mg) reacts completely with oxygen? (Atomic masses: Mg=24, O=16) 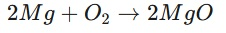
A. 16g
B. 20g
C. 40g
D. 80g
C. 40g
A lift raises a 600 kg load vertically through a height of 5 m in 10 seconds. Calculate the power developed by the lift. (Take g = 10 N/kg)
A. 300 W
B. 3000 W
C. 30,000 W
D. 300,000 W
B. 3000 W
Which of the following statements about the role of xylem in plants is correct?
A. It transports sugars from the leaves to other parts of the plant.
B. It transports water and mineral ions from the roots to the leaves.
C. It is responsible for the exchange of gases during photosynthesis.
D. It stores excess glucose for future use.
B. It transports water and mineral ions from the roots to the leaves.
Which of the following correctly describes the relationship between current, voltage, and resistance in a series circuit?
A. Current is divided, voltage is the same, resistance is added.
B. Current is the same, voltage is divided, resistance is added.
C. Current is divided, voltage is divided, resistance is added.
D. Current is the same, voltage is the same, resistance is added.
B. Current is the same, voltage is divided, resistance is added.
If the bond energies for breaking bonds are greater than the bond energies for forming bonds in a chemical reaction, the reaction is:
A. Exothermic, with a positive energy change.
B. Exothermic, with a negative energy change.
C. Endothermic, with a positive energy change.
D. Endothermic, with a negative energy change.
C. Endothermic, with a positive energy change.
Which process is primarily responsible for returning carbon from the atmosphere to plants?
A. Respiration
B. Combustion
C. Photosynthesis
D. Decomposition
C. Photosynthesis
What type of reaction occurs when an acid reacts with an alkali?
A. Precipitation reaction
B. Redox reaction
C. Neutralisation reaction
D. Displacement reaction
C. Neutralisation reaction
When extracting DNA from fruit, a common step involves adding a detergent (like washing-up liquid). What is the primary role of the detergent in this process?
A. To denature the DNA, making it visible.
B. To break down the cell walls and membranes.
C. To precipitate the DNA out of solution.
D. To digest proteins associated with the DNA.
B. To break down the cell walls and membranes
What is the primary purpose of 'cracking' in the oil industry?
A. To separate crude oil into its different fractions.
B. To break down larger, less useful hydrocarbon molecules into smaller, more valuable ones.
C. To remove impurities from crude oil.
D. To combine small hydrocarbon molecules to form larger ones.
B. To break down larger, less useful hydrocarbon molecules into smaller, more valuable ones.
Two populations of a species are geographically isolated. Over time, they evolve into two distinct species. This process is known as:
A. Adaptive radiation
B. Allopatric speciation
C. Sympatric speciation
D. Co-evolution
B. Allopatric speciation
Why is ionising radiation dangerous to living organisms?
A. It causes the body to overheat rapidly.
B. It can disrupt chemical bonds and damage DNA.
C. It leads to the immediate formation of new radioactive elements in the body.
D. It only affects the nervous system, leading to paralysis.
B. It can disrupt chemical bonds and damage DNA.
If the activity of a radioactive sample decreases from 800 Bq to 100 Bq in 30 minutes, what is the half-life of the sample?
A. 5 minutes
B. 7.5 minutes
C. 10 minutes
D. 15 minutes
C. 10 minutes
A reaction produces 8.8g of carbon dioxide (CO2) from 4.8g of carbon. What is the percentage yield if the theoretical yield of CO2 is 17.6g?
A. 25%
B. 50%
C. 75%
D. 100%
B. 50%
Which of the following scenarios involves negative work being done?
A. A force pulling a box across a floor.
B. A book falling under gravity.
C. Friction acting on a moving object.
D. A spring being compressed by an external force.
C. Friction acting on a moving object.
Which process in plants is primarily responsible for the upward movement of water against gravity in the xylem?
A. Translocation
B. Active transport
C. Transpiration pull
D. Osmosis through root hairs
C. Transpiration pull
A household appliance has a power rating of 2 kW. If it is used for 3 hours, how much energy (in kWh) does it consume?
A. 0.6 kWh
B. 2 kWh
C. 3 kWh
D. 6 kWh
D. 6 kWh
A student investigates the temperature change during the dissolution of ammonium nitrate in water. They observe a significant drop in temperature. This observation indicates that the dissolution is:
A. An exothermic process, absorbing energy from the surroundings.
B. An exothermic process, releasing energy to the surroundings.
C. An endothermic process, releasing energy to the surroundings.
D. An endothermic process, absorbing energy from the surroundings.
D. An endothermic process, absorbing energy from the surroundings.
Eutrophication in aquatic ecosystems is primarily caused by an excess of which nutrients?
A. Oxygen and carbon dioxide
B. Nitrogen and phosphorus
C. Sulfur and chlorine
D. Potassium and calcium
B. Nitrogen and phosphorus
Which of the following is the most accurate statement about a strong acid?
A. It completely dissociates in water to produce hydrogen ions.
B. It only partially dissociates in water.
C. It has a pH of 4
D. It reacts slowly with metals.
A. It completely dissociates in water to produce hydrogen ions.
After adding cold ethanol to the filtered fruit extract during DNA extraction, the DNA often appears as a white, stringy precipitate. Why is cold ethanol used, and why does the DNA precipitate?
A. Cold ethanol dissolves DNA, and the low temperature causes it to solidify.
B. Cold ethanol denatures proteins, and the cold helps to keep the DNA intact.
C. DNA is insoluble in cold ethanol, and the cold temperature further reduces its solubility, causing it to clump together.
D. Cold ethanol acts as a catalyst, and the precipitation is a result of a chemical reaction.
C. DNA is insoluble in cold ethanol, and the cold temperature further reduces its solubility, causing it to clump together.
Which of the following is a characteristic property of alkanes?
A. They contain carbon-carbon double bonds.
B. They are generally very reactive due to their double bonds.
C. They are saturated hydrocarbons, meaning they contain only carbon-carbon single bonds.
D. They readily undergo addition reactions.
C. They are saturated hydrocarbons, meaning they contain only carbon-carbon single bonds.
Which of the following statements about mutations is most accurate in the context of evolution?
A. All mutations are harmful to an organism.
B. Mutations always lead to the formation of new species.
C. Mutations are the ultimate source of new genetic variation.
D. Mutations occur in response to environmental pressures.
C. Mutations are the ultimate source of new genetic variation.
In a nuclear power plant, which of the following processes is used to release energy from radioactive materials?
A. Nuclear fusion
B. Alpha decay
C. Nuclear fission
D. Beta decay
C. Nuclear fission
A graph shows a linear relationship between two variables, x and y. If the gradient of the line is 3 and the y-intercept is 5, what is the value of y when x = 10?
A. 35
B. 45
C. 15
D. 25
A. 35
In the reaction between nitrogen and hydrogen to form ammonia: N2+3H2→2NH3. If 14g of nitrogen reacts completely, what mass of hydrogen is needed? (Atomic masses: N=14, H=1)
A. 1g
B. 3g
C. 6g
D. 9g
B. 3g
A motor lifts a mass of 20 kg to a height of 15 m. If the motor's efficiency is 75% and the total energy supplied to the motor is 4000 J, calculate the work done against gravity. (Take g = 10 N/kg)
A. 3000 J
B. 3200 J
C. 3500 J
D. 3750 J
A. 3000 J
The rate of transpiration in plants is influenced by several environmental factors. Which of the following conditions would lead to the lowest rate of transpiration?
A. High light intensity and high temperature.
B. Low humidity and high wind speed.
C. High humidity and low temperature.
D. High light intensity and low humidity.
C. High humidity and low temperature.
Which of the following statements about parallel circuits is true?
A. Adding more resistors in parallel increases the total resistance.
B. The current is the same through each branch.
C. The voltage across each component is the same.
D. If one component fails, the entire circuit breaks.
C. The voltage across each component is the same.
Consider the reaction profile diagram for a reversible reaction. If the forward reaction is exothermic, which of the following statements about the reverse reaction is correct?
A. The reverse reaction is also exothermic and has a higher activation energy.
B. The reverse reaction is endothermic and has a higher activation energy than the forward reaction.
C. The reverse reaction is endothermic and has a lower activation energy than the forward reaction.
D. The reverse reaction is exothermic and has the same activation energy as the forward reaction.
B. The reverse reaction is endothermic and has a higher activation energy than the forward reaction.
Which of the following processes plays a crucial role in converting atmospheric nitrogen gas into a usable form for plants?
A. Denitrification
B. Respiration
C. Nitrogen fixation
D. Combustion
C. Nitrogen fixation
When an acid is titrated with an alkali, what piece of apparatus is used to accurately measure the volume of alkali added to the acid in the conical flask?
A. Measuring cylinder
B. Pipette
C. Burette
D. Beaker
C. Burette