This states that matter cannot be created nor destroyed.
What is Law of Conservation of Matter?
This is found on the left side of the reaction formula. In other words, what goes in to the reaction.
What is reactant?
There are four atoms of carbon before a chemical reaction. How many should there be after?
What is four?
Name of protective eyewear used during labs.
What is goggles?
The smallest unit of an element.
What is atom?
The masses of the reactants and the products in a chemical reaction according to the Law of conservation of mass should always be ____.
What is equal?
It is the end result of a chemical reaction.
What is product?
A glass contains 200g of water. A student adds 35g of sugar and mixes it up. What is the mass of the mixture?
What is 235g?
The name of this glassware. 
What is beaker?
Two or more atoms bonded together.
What is molecule?
True or false: during a chemical reaction, atoms are rearranged.
What is true?
In a chemical equation, the mass of the reactants is ________ to the mass of the products.
What is equal?
How many atoms of carbon (yellow) are there on the product side of this reaction?
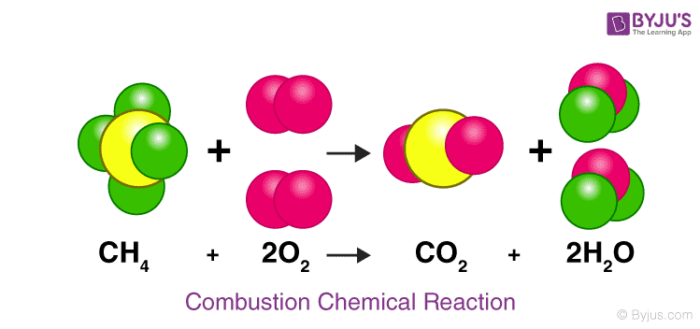
What is one?
Name of lab equipment used to measure liquids; shown below. 
What is graduated cylinder?
Matter is anything that has _____ and _____ .
What is mass and takes up space.
Twelve grams of reactant X react with fifteen grams of reactant Y. This is the total mass of the product(s).
What is twenty seven (27) grams?
True or false: the Law of Conservation of Matter applies only to physical reactions, not chemical reactions.
What is false?
How many atoms of oxygen (pink) are there on the reactant side of this reaction?

What is four?
The unit used for mass in science.
What is g (grams)?
The unit used for liquids in science.
What is mL (milliliters)?
In a chemical reaction, 50 g of Chlorine is used to produce 70 grams of Sodium Chloride. How much Sodium was used?
What is 20 grams?
This gas is produced from the reaction between vinegar and baking soda.
What is carbon dioxide?
The mass of the products of a reaction are 100g. What are possible masses of reactants X, Y, and Z?
What is 20g, 20g, 60g? (50g, 25g, 25g) etc.
Name of this glassware. 
What is flask?
Two pieces of evidence that a chemical change is happening to a substance.
What are color change/temperature change/new substance is formed/bubbling?