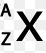Solubility (dissolves)
What is physical property?
What happens to the atoms of a substance in a physical change?
What is Atoms change their arrangement and motion?
What kind of physical property is this? Volume
What is extensive physical property?
Which of the following determines the identity of an element?
What is atomic number?
Stretched rubber band is an example of ____ energy.
What is potential?
The ability to burn is known as---
What is flammability
What is physical change?
What kind of physical property is States of Matter
What is intensive physical properties?
The average mass of all the isotopes of an element
What is atomic mass?
The mass of a substance divided by its volume.
What is density?
food color added to milk is _____ property.
what is a physical property
A piece of metal is left in the rain and forms rust.
What is chemical change?
Properties that DO depend on the amount of matter present.
What is extensive physical property?
electrons have what charge?
What is negative?
A phase change from a liquid to a gas is called?
What is boiling?
Reacts with acid
What is chemical property?
extracting iron filings from a sand mixture using a magnet
What is a physical change?
What kind of physical property is this? Odor
What is intensive?
The least amount of mass is located in the ....
What is the electron cloud?
CO is an
What is a compound?
Which use of iron is due to its chemical properties
What is producing colored sparks in fireworks?
Wood is burned in a campfire and is turned into ash and smoke.
What is a chemical change
What kind of physical property is this? Luster
What is intensive?

What is neutron?
In a correctly written symbol what would be located in the "A" position?
What is mass number?