Elements of Group 1A are called?
Alkali Metals
Who is smaller?
Lithium or Fluorine
Fluorine
Who is Larger
Chlorine or Sodium
Chlorine`
What is Electronegativity?
A measure of the tendency of an atom to attract a bonding pair of electrons.
Which atom has a larger radius?
Cl-1 ion
Cl
Cl-1
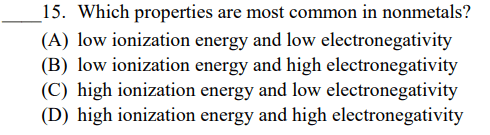
D
Their are two NFL teams in LA, who are they?
Rams
Chargers
What is the Memphis NBA team called?
Memphis Grizzles

Coldplay

Clemson
How many Groups are there in the Periodic Table?
18
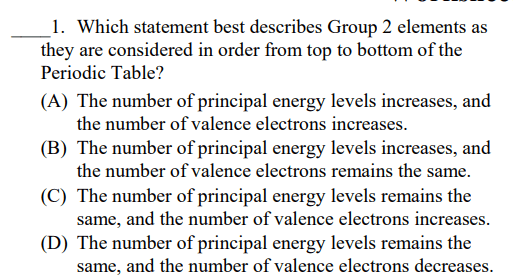
Elements of Group 2A are called?
Alkaline Earth Metals
Who is larger
Lithium or Francium
Francium
Who has a smaller Ionization Energy?
Francium or Lithium
Francium
Who has a larger electronegativity?
Oxygen or Nitrogen
Oxygen
What happens to the size of an atom when it becomes a cation? Why?
Cations are atoms that have LOST electrons to become POSITIVELY charged. This causes an increase in the proton-electron ratio, increasing the coulombic attraction.
D
This singer sings, Firework, Teenage Dream, Roar and Waking Up in Vegas
Katy Perry
Who is the current speaker of the House of Represenative?
Mike Johnson

The Killers

University of Miami
Elements of Group 7A are called?
Halogens
Magnesium or Calcium
Magnesium
What is Ionization Energy?
Energy required to remove an electron, and become an ion
Who has a larger electronegativity?
Bromine or Iodine
Bromine
Arrange each group of elements in order of increasing ionization energy.
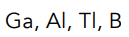


C
What is the capital of Ukraine?
Kyiv
The professional Lacrosse team in Philadelphia is called the ________________.
Wings
Everytime
Lucky
Overprotected
Oops I Did It Again
If You Seek Amy
Britney Spears

University of Arizona
Elements of Group 8A are called?
Noble Gases
Who is Larger
Scandium or Chromium
Scandium
Who has a smaller Ionization Energy?
Barium or Magnesium
Barium
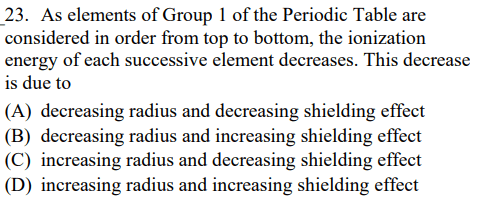
Metals have a ____________ electronegativity, while non-metals have a _____________ electronegativity.
Weak
Strong

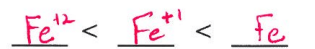

A
He is the Head Coach of what team?
SF 49ners
Emmanuel Macron


University of Stanford
Which scientist came up with the "Law of Octaves"
John Newlands
Elements of Group 6A are called?
Chalcogens
Who is Larger?
Iodine or Chlorine
Iodine
Which atom has a larger Ionization Energy?
1s22s22p63s1 or 1s22s22p63s2
1s22s22p63s2
Noble Gases have an electronegativity value of ___________
0
What does the Electron Affinity refer to?
Electron affinity is defined as the change in energy (in kJ/mole) of a neutral atom (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Life
:max_bytes(150000):strip_icc():focal(749x0:751x2):format(webp)/king-charles-012624-2-9cd6206f354d4e72bce30af1bb8b91af.jpg)
King Charles

Elton John

Syracuse