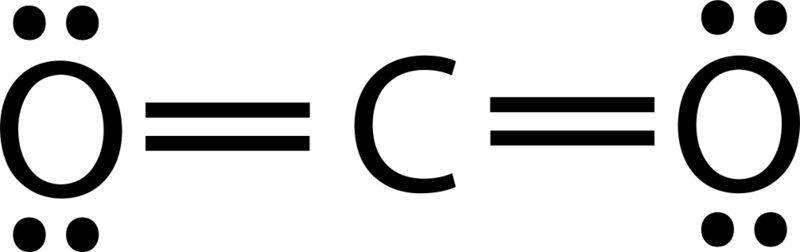Number of Valence electrons in the first column
1
Lewis dot for Hydrogen
H with one dot
The Lewis Dot Structure of CH4
4 h around 1 c
What type of bond is formed between Lithium and Fluorine?
Ionic
What is the molecular geometry and bond angle for CO2?
Linear, 180o
Number of Valence electrons in group 13
3
Lewis dot for O
6 dots around O
The Lewis Dot Structure of NH3
3 h around 1 n with 2 paired electrons
What type of bond is formed between Magnesium and Sulfur?
Polar Covalent
What is the bond angle and shape for the molecule below?

Trigonal Planar 120o
Number of Valence electrons in group 16
6
The Lewis Dot Structure of Antimony.
5 dots around Sb
Lewis Dot Structure for HCl
h-cl 3 sets of paired electrons
What type of bond is formed between Phosphorous and Sulfur?
Give the molecular geometry and bond angle for the molecule below:
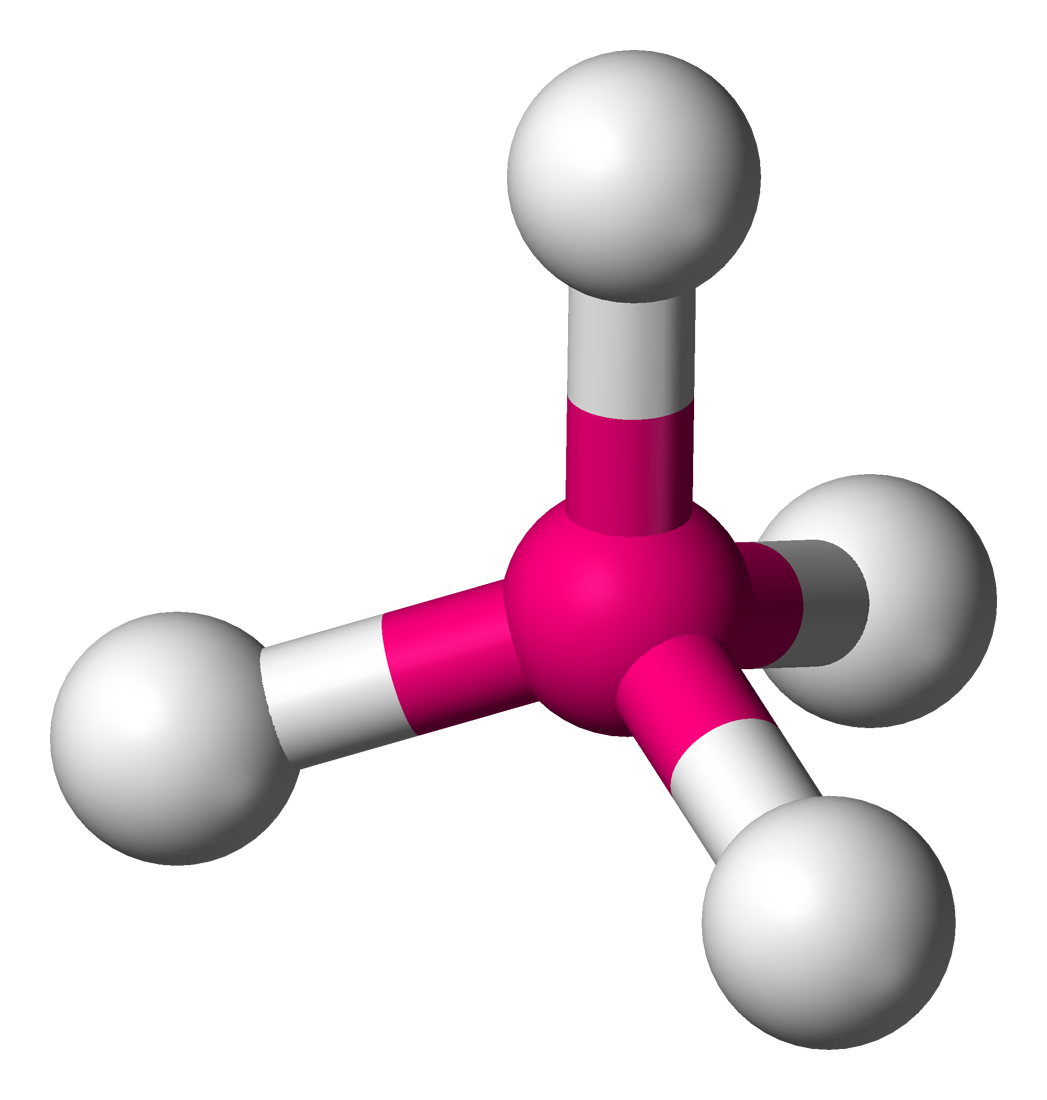
Tetrahedral, 109.5o
Number of Valence electrons in group 2A
2
The Lewis Dot Structure for Chlorine (Cl).
7 dots around Cl
The Lewis Dot Structure of H2O
h-o-h 2 paired electrons on o
What type of bond is formed between Sodium and Chlorine?
Ionic
What is the molecular geometry for NF3?
Trigonal Pyramidal
Number of Valence electrons in group 18 (Include the exeption).
8 except for He which has only 2
The Lewis Dot Structure of Selenium (Se).
6 dots around Se
The Lewis Dot Structure for HCN
1 h single bond c triple bond n 1 paired electrons
What type of bond is formed between Hydrogen and Carbon?
Polar Covalent
If a compound consists of 3 atoms and has a bond angle of 109.5. What is its molecular geometry?
Bent