Draw the Lewis structure
Lewis with charge
Find what's wrong
100
O2

100
OH-
100
should be 16 electrons total
there are 18 electrons shown in this model
200
C2H6
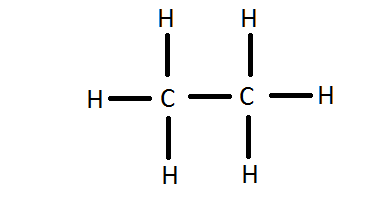
200
BH2-
200
O should be the central atom! This structure is just wrong...
300
CF4

300
NO3-
300
none of the Os have 8 electrons, and they don't have 6 electrons for themselves.
400
N2H4
400
NH4+
400
F does not have the electrons to give N, it should have only one bond because it wants to have 7 electrons for itself.
500
C2H3N

500
ClF4+
500
N does not have 8 electrons shared