The first step taken when approaching a Lewis Structure problem
What is Calculating the total number of valence electrons of the compound.
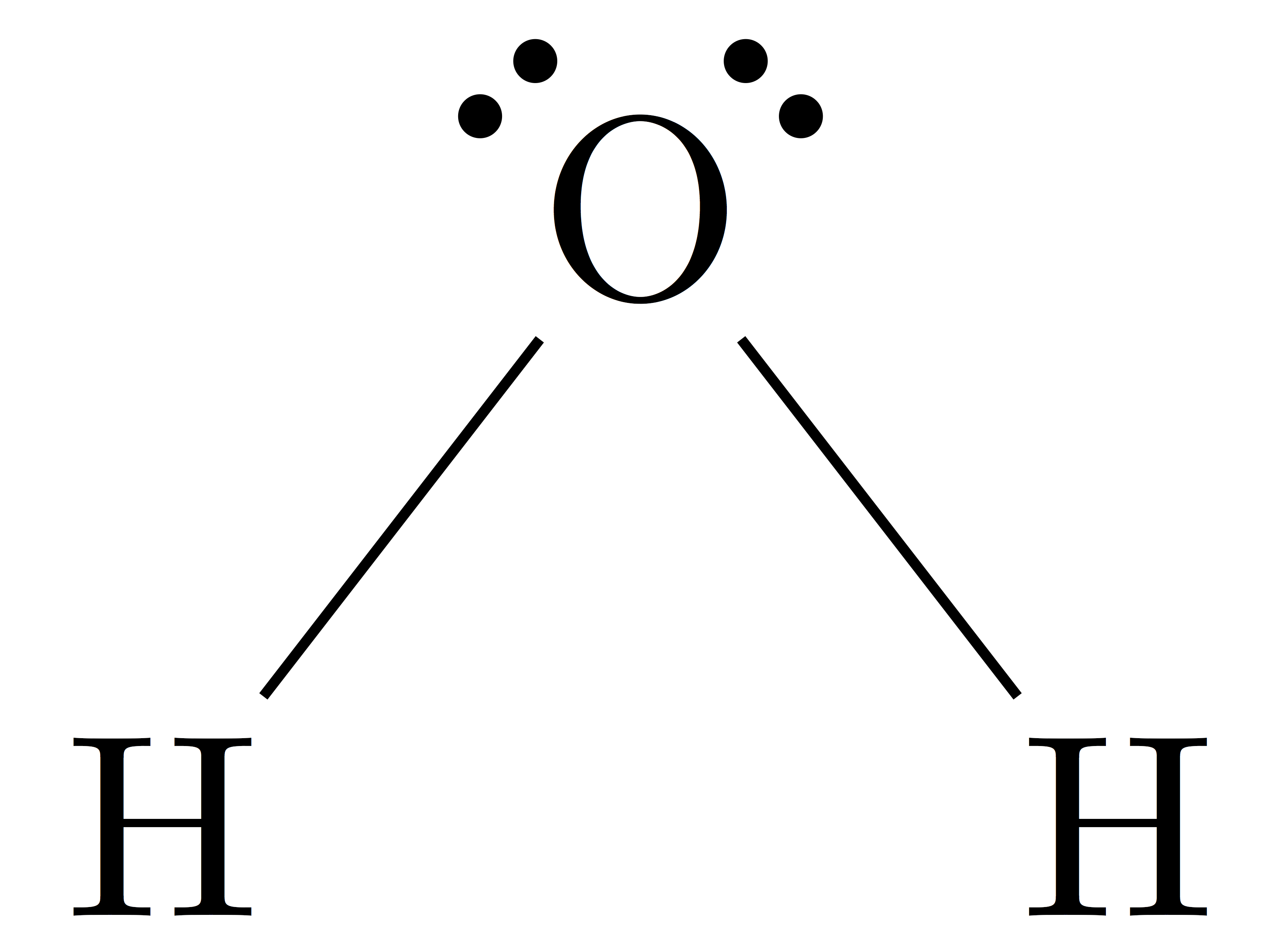
The Lewis Structure for water (H2O)
How do you know the number of bonding domains?
How do you know the number of non-bonding domains (or lone pairs)?
Bonding domains - you count the number of bonds for the central atom.
Non-bonding domains/lone pairs - you count the number of lone pairs (or electrons outside of bonds in pairs) for the central atom.
The equation for formal charge
What is Formal charge = # valence shell electrons - (1/2) bonding electrons - # of lone pair electrons
The second step taken when approaching a Lewis Structure problem
What is drawing the basic structure and bonds between the atoms.
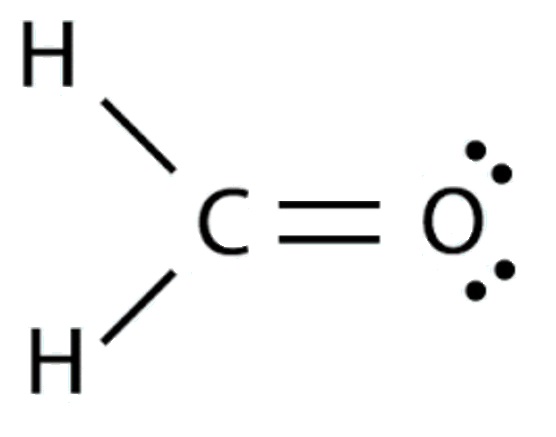
The Lewis Structure for formaldehyde (CH2O)
The electron domain geometry and molecular geometry for XeF4
What is Octahedral (electron domain geometry) and Square Planar (molecular geometry)
The definition of resonance structure
What is two or more Lewis structures with the same arrangement of atoms can be written for a molecule or ion
The third step taken when approaching a Lewis Structure problem
What is adding remaining electrons to atoms (that are not the central atom).
The Lewis Structure for Hexachlorocyclohexane (HCCH)
The electron domain geometry and molecular geometry for ClF3
What is trigonal bipyramidal (electron domain geometry) and T-shaped (molecular geometry)
The definition of nonpolar covalent bonds
What is the equal sharing of electrons between nonmetals
The fourth step taken when approaching a Lewis Structure problem
What is looking at the octet rule and making sure all atoms follow the rule (unless there is an exception!)
The electron domain geometry and the molecular geometry for TeCl42−
What is octahedral (electron-pair geometry) and square planar (molecular structure)
The definition of ionic bonds
What is the complete transfer of electrons (between metals and nonmetals)
The final step taken when approaching a Lewis Structure problem
What is calculating the formal charge and ensuring all charges are minimized (or optimized).
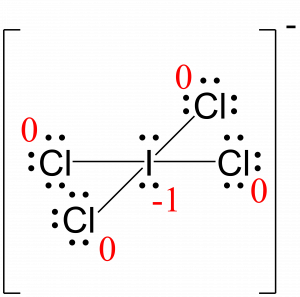
The Lewis Structure for ICl4-
The electron domain geometry and molecular structure for PH2−
What is tetrahedral (electron domain geometry) and bent (molecular geometry)
The definition of polar covalent bonds
What is the unequal sharing of electrons between two nonmetals