What is the shape name and bond angles of AX3E1?
Trigonal Pyramidal.
107°
Electrons on the outermost shell or energy level
What are valence electrons
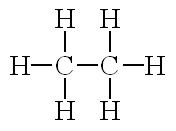
What is the correct Lewis Structure for C2H6?
What number are you trying to be the closest to when calculating formal charge.
0.
Which Step is first when drawing Lewis Structures?
Complete the octet for all atoms.
Check to see if there are too many electrons.
Complete the octet for all atoms. (Remember exceptions)
What is the shape name and bond angles of AX5?
Trigonal Bipyramidal
120° and 90°
A pair of electrons that is not involved in bonding and belongs exclusively to one atom.
What is a lone pair
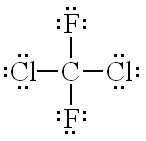
What is the correct Lewis Structure for CCl2F2?
Oxygen has 6 valence electrons, 1 lone pair, and 3 bonds; what is its formal charge?
6-2-3=1
How many bonds does Beryllium make?
2 bonds.
What is the shape name and bond angles of AX6?
Octahedral.
90°
One of two or more Lewis Structures for a single molecule that can be drawn to represent a molecule.
What is a resonance structure
What compound does this Lewis Structure Represent?
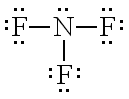
NF3
Calculate the charge of C and Cl in CCl4.
C: 4-0-4=0
Cl: 7-6-1=0
How many bonds does Boron need?
3 Bonds.
What is the shape name and bond angles of AX5E1?
Square Pyramidal.
>90°
An electron configuration notation with only the valence electrons of an element shown, indicated by dots placed around the element’s symbol.
What is a Lewis Dot Diagram
Which of the following contains the correct Lewis structure of a compound with one Si and four Br atoms?
A:
B:
C: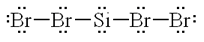
D:
B: 
What is the formal charge of C,O, and H?
:max_bytes(150000):strip_icc()/formaldehyde_LD-56a12a2c3df78cf772680353.png)
C: 4-0-4=0
O: 6-4-2=0
H: 1-0-1=0
What do you do if you draw your Lewis Structure and there are too many electrons?
Make double and triple bonds.
What is the shape name and bond angles of AX3E2?
T-Shaped.
90° and 180°
What is an isomer?
Two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
What are the three resonance structures for CO32-?
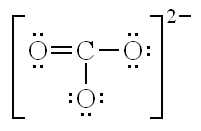
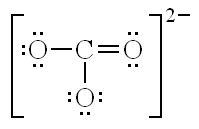
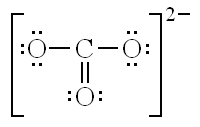
What is the equation for finding the Formal Charge of an atom?
What do you do if you draw your Lewis Structure and there are too few electrons?
Make an expanded octet.