This type of light has the highest frequency.
What is, gamma radiation?
The symbol for frequency, and what it's called...
What is, v, called "nu"?
When light has a large wavelength, its frequency will be this.
What is, low?
The equation for energy problems.
What is E=hv ?
This effect may occur if light carrying enough energy is able to eject electrons from the surface of metal.
What is, the Photoelectric Effect?
The type of electromagnetic radiation has the longest wavelength.
What is, radio waves?
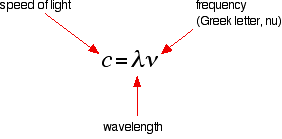
The symbol for the speed of light.
What is, "c"?
Multiplying wavelength and frequency will give this.
The value of h is...
What is 6.626×10-34 J s?
An electron in its normal orbit is said to be at this.
What is "ground state"?
The order of VISIBLE light from longest wavelength to shortest.
What is, red, orange, yellow, green, blue, indigo, violet?
The symbol for wavelength, and what it is called.
What is lambda?
The 3 forms of light with lower frequency than visible light.
What are radio, microwave, and infrared?
The unit for energy
What is, joules (J)?
When excited light from an element is passed through a prism, it produces a unique...
What is, line emission spectrum?
The form of light in this list that has the shortest wavelength:
x-ray, radio waves, infrared
What is, x-ray?
What is always the value for c?
3.00x108 m/s
Light that has higher frequency than blue light but less than ultraviolet.
What is, violet (or purple)?
The energy equation, rearranged to solve for frequency.
What is ...
E/h
Light can only be emitted by an electron when this occurs.
When the electron falls from excited to ground state.
The order of light forms on the electromagnetic spectrum, from HIGHEST energy to LOWEST energy.
What is--gamma, x rays, ultraviolet, visible light, infrared, microwaves, and radio waves?
The equation used to show the relationship between wavelength and frequency.
What is...?
The 3 forms of light that have shorter wavelengths than visible light.
What are, ultraviolet, x-ray, and gamma?
Light with a frequency of 4.35 x 1014 Hz carries energy. This is the calculation you would do to find that energy.
E = (6.626 x 10-34 Js) (4.35 x 1014 1/s)
The more energy levels an electron jumped when absorbing light, the ___________ the frequency will be of the emitted light released from the falling electron.
The form of light we can see.
What is, Visible?
Of the two, the frequency associated with the longer wavelength:
1.2 x 1014 Hz or 1.2 x 1018 Hz
What is, 1.2 x 1014 Hz?
Lower frequencies have longer wavelengths.
In this pair, the wavelength of light that has the shorter frequency:
450 nm or 700 nm
What is, 700 nm?
Longer wavelengths mean shorter frequencies.
The unit that is the same as Hertz.
What is 1/s or s-1 ?
Note: "s" is NOT correct.
The type of light that would cause the greatest jump in energy levels for an electron.
What is, Gamma?
Gamma carries the most energy and could move an electron more than less energetic forms of light.