A specific type of dipole-dipole interaction that involves H to be bonded to N, O, or F.
What is hydrogen bond?
The strongest intermolecular force that this molecule, HF, can participate in.
What is hydrogen bonding?
The name(s) for the transition labeled 2.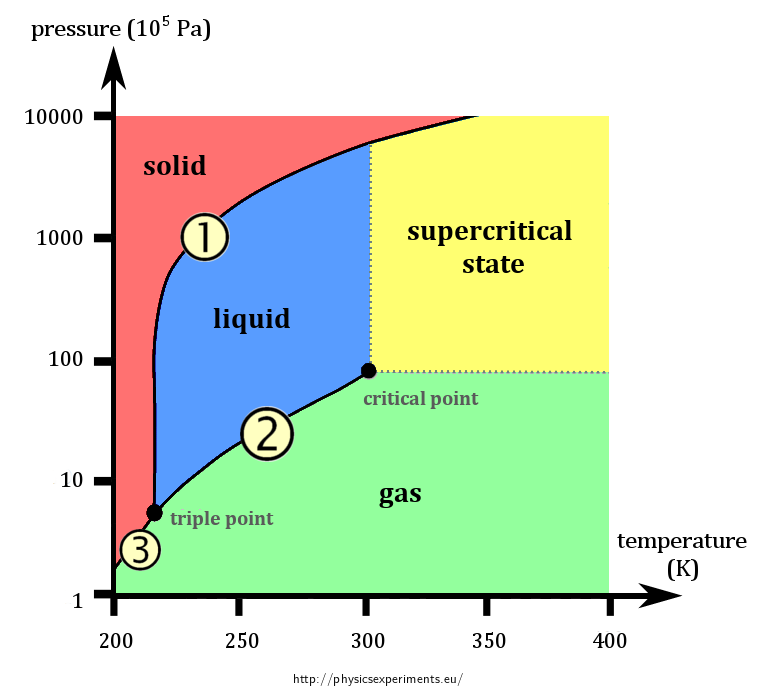
What is evaporation?
Either water (H2O) or carbon dioxide (CO2) have a higher boiling point.
What is water?
Term for the measure of how easily liquid flows.
What is viscosity?
An intermolecular interaction that involves some sort of polar molecule overwhelming a charged ion.
What is ion-dipole interaction?
The weakest intermolecular force that these reagents experience with each other.
CH3CHOHCH3 + NaCl -> CH3CHOHCH3 + Na+ + Cl-
What is London dispersion force?
There is a term for a specific pressure and temperature where all three states of matter of a substance exist in equilibrium.
If intermolecular forces in a liquid are stronger, the liquid will tend to either be more or less viscous than a liquid with weaker intermolecular forces.
What is more viscous?
What is cohesive forces?
An intermolecular force caused by a short-lived, spontaneous dipole moment.
What is London dispersion force?
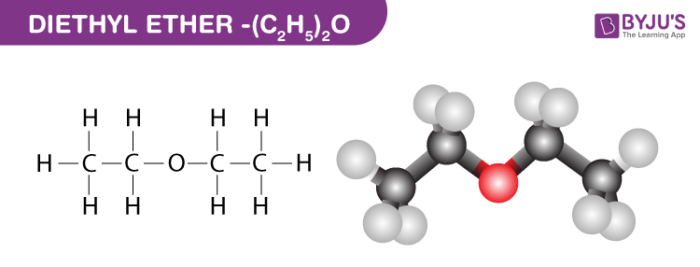 All of the intermolecular forces that diethyl ether can participate in.
All of the intermolecular forces that diethyl ether can participate in.
What are dipole-dipole interactions, and London dispersion forces?
There is a word that describes changing from a solid to a gas.
What is sublimation?
Either pentane, 2-methylbutane or neopentane (all have the formula C5H12) has a higher boiling point than the other.

What is pentane?
Describes intermolecular forces between a substance and a surface.
What is adhesive forces?
An intermolecular force caused by differences in electronegativity between atoms in a molecule.
What is dipole-dipole interaction?
All of the intermolecular interactions experienced in a mixture of ammonia (NH3) and water (H2O).
What are hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
CO2 is initially at 6.0 atm and -100 Celsius, then is in a certain phase after it is heated to 25 Celsius and pressurized to 10 atm. 
What is gas?
There is an order in which the following molecules are arranged in increasing boiling point.
H2O, Xe, H2S, MgCl2, N2
What is N2, Xe, H2S, H2O, MgCl2
A substance reaches dynamic equilibrium between its liquid and gaseous phases at this.
What is vapor pressure?
Rank the intermolecular forces from weakest to strongest.
What is London dispersion forces, dipole-dipole interactions, hydrogen bonding, and ion-dipole interactions (and ionic bonding... sort of)?
All of the molecular forces that the leaving group, benzyl tosylate, can participate. 
What are dipole-dipole interaction, and London dispersion forces.
Carbon dioxide undergoes a process as it is cooled from 25 Celsius to -80 Celsius at 1 atm. 
What is deposition?
One of these molecules have the highest viscosity at room temperature.
Water: H2O
Glycerol: HOCH2CH(OH)CH2OH
Ammonia: NH3
Pentane: CH3(CH2)3CH3
Dipropyl ether: CH3CH2CH2OCH2CH2CH3
What is glycerol?
This is a term that describes the likelihood an induced dipole occurs in an atom or molecule.
(This trends down the periodic table)
What is polarizability?