Ionic compounds create shapes called crystal lattices because of the electrostatic force of attraction that exist between the cations.

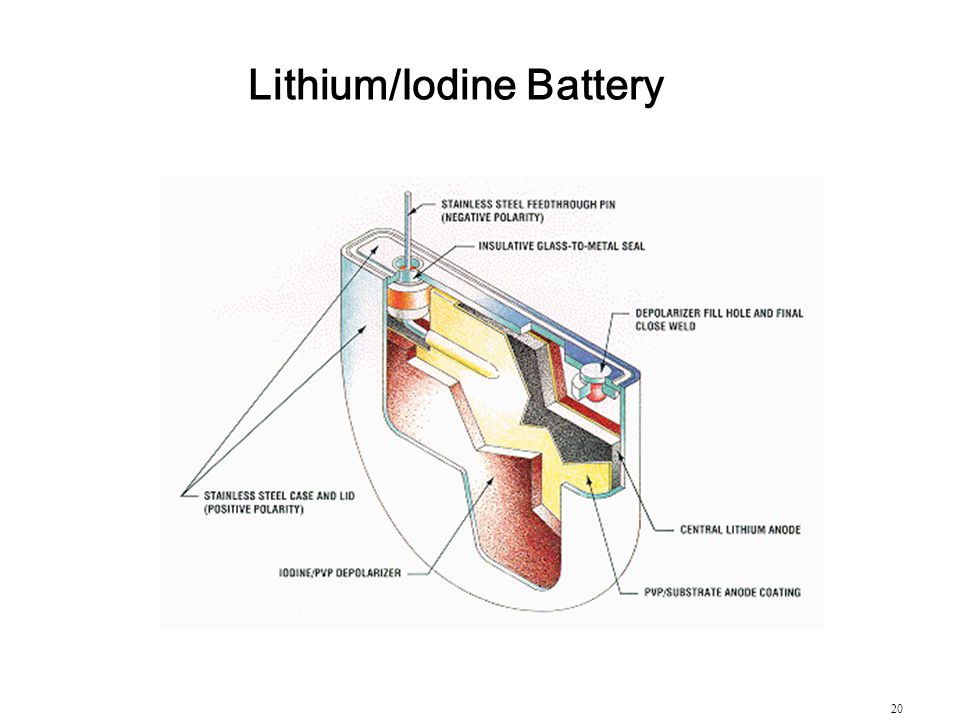
- The boiling point is 1,171 degrees Celsius and the melting point is 469 degrees Celsius .
- The appearance of Lithium Iodide is said to be a white crystalline solid. When its exposed to air, it becomes yellow in colour, due to the oxidation of iodide to iodine.
- Lithium Iodide is very reactive due to the reaction of lithium which is a part of the Alkali metals and iodine, a part of the halogens.
- It is said that the rigidity in a solid compound is very high, hence the fact that Lithium Iodide has high rigidity.
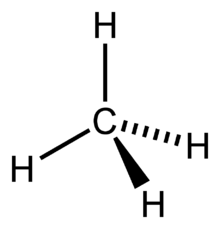
Molecular compounds create a type of stereochemical shape called a tetrahedral. Tetrahedral's have one central atom and four other peripheral atoms around it.


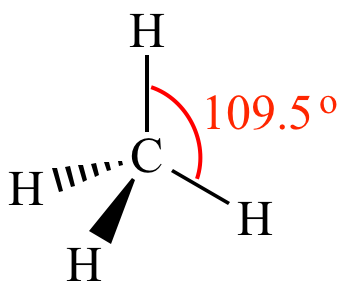

- As for the shielding of Lithium Iodide, because of lithium giving iodine its electron, it creates a a full octet on the fifth orbital of iodine. This creates a bigger radius for iodine which results in many orbitals which reduces the electron attraction from the nucleus.

As for Octane, it has many structural bonding patterns and atomic organization that differ by the amount and location of branching in the carbon chain.