What number does "deca-" represent?
10.
is it easier for "Ra" to gain 6 or loose 2 valence electrons
Loose 2 valence electrons
which orbital block is "Nickel" in?
D block
what is Transform?
plates sliding past one another
Which option represent milli?
m_
or
_m/m
_m/m
5
Is it easier for "Cs" to gain or lose electrons?
Lose.
what orbital block is "Tellurium" in?
P block
what is divergent
plates are moving away
What are surroundings in a system?
Everything else in the Universe.
how many valence electrons does carbon need to be stable
4
Al+3 + N-3 --> Al N
How many "Al" and "N" are there?
1 Al and 1 N
what period is "Zinc" in?
what are Transform plate boundarys
lateral sliding
What is nuclear energy?
Potential energy stored in Nucleus of Atom.
On the right image, what do the lines in between both "N" represent?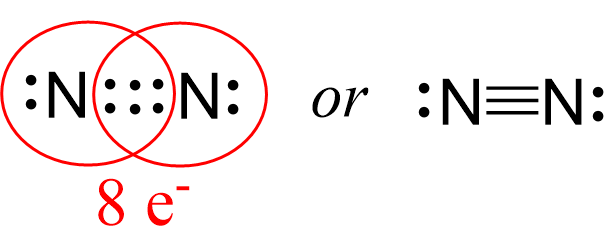
Covalent bonds.
What is a Cation and Anion?
Cation- lose valence electrons.
Anion- gain valence electrons.
what is the outermost subshell of "sulfur".?
3p4
What is happening here?
Subduction.
What is Endothermic?
absorption of heat.
Name the following covalent compounds:
C4H10
Tetracarbon Decahydride.
How many "Ra" and "At" would you need if Ra and At bonded
1 "Ra" and 2 "At"
what is the electron configuration for "Cesium"?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1
what does subduction produce on the surface
Volcanoes
What is chemical energy?
Potential energy stored in chemical bonds of compounds.